The Cardiac Autonomic Control System Response to Submaximal Exercise Test in Children with Cerebral Palsy Compared to Typical Peer
Taly Amichai and Michal Katz-Leurer*
DOI10.36648/2472-1786.5.2.84
Taly Amichai and Michal Katz-Leurer*
Department of Physical Therapy, Sackler Faculty of Medicine, School of Health Professions, Tel-Aviv University, Israel
- *Corresponding Author:
- Michal Katz-Leurer
Department of Physical Therapy
Sackler Faculty of Medicine
School of Health Professions
Tel-Aviv University
Tel-Aviv 69978, Israel
Tel: 972-3-6405432
Fax: 972-3-6409223
E-mail: michalkz@post.tau.ac.il
Received Date: April 22, 2019; Accepted Date: May 15, 2019; Published Date: May 24, 2019
Citation: Amichai T, Katz-Leurer M (2019) The Cardiac Autonomic Control System Response to Submaximal Exercise Test in Children with Cerebral Palsy Compared to Typical Peer. J Child Dev Disord. Vol.5 No.2:6.
Abstract
Objective: To compare the cardiac autonomic system at rest and its response to a submaximal aerobic test in children with cerebral palsy (CP) and typically developed (TD) controls.
Design: Twenty-five children with CP aged 6-11 and 20 age and gender matched TD controls participated in the study. RR intervals were monitored at rest, while performing the sub-maximal treadmill test, and during the recovery period. The square-root of the mean of successive differences between adjacent RR intervals (RMSSD) was calculated.
Results: The median level of the submaximal treadmill test stage was 3 for children with CP (95% CI 0.75-3.25), 16/20 TD children completed all test stages. The Log- Rank statistic (χ2 1=30.4) was highly significant (p<0.001). At rest the RMSSD values in children with CP were significantly lower as compared to children TD, and changed less due to the submaximal test and recovery stages (interaction-effect, F2;86=9, p<0.01).
Conclusion: Children with CP show lower RMSSD at rest, and less adaptive to exercise as compared to TD children. Performing physical activity is highly recommended for children with CP, re-educating the cardiac autonomic system is one of its main goals. Assessing the autonomic response to different exercise protocols is the next step needed.
Keywords
Cerebral palsy; Cardiac autonomic system; Submaximal test
Introduction
Cerebral palsy (CP) caused by a brain insult prior to, during, or shortly after birth is the leading cause of physical neurodevelopmental disability in the western world [1]. The primary neuro-motor impairments cause secondary impairments such as spasticity, weakness, and decreased range of motion, all of which contribute to the more sedentary behavior of children and adults with CP [2].
In the last two decades, few studies have described the signs of autonomic impairment in children with CP [3], one of which is higher heart rate (HR) at rest, corresponding to the 90th percentile of age matched typically developed (TD) children, as well as significantly lower heart rate variability (HRV) measures [3-6].
HRV is a statistical measure of the RR oscillations over time, which reflects the dynamic response of the cardiac autonomic system to physiological changes. HRV can be assessed through different methods, one of which is time domain measures which can include the standard deviation of all RR intervals (SDNN) or the square root of the mean squared differences of successive differences (RMSSD) [7].
The cardiac autonomic imbalance in children with CP might be due to the primary brain insult [8] or to a more sedentary lifestyle, or both. In TD children and healthy adults, performing regular physical activity can modify the cardiac autonomic balance by increasing parasympathetic and decreasing sympathetic activity at rest [9-11]. These changes are evident in the reduction of HR and an increase of HRV parameters at rest. Promoting physical activity is a main goal in the rehabilitation process of children with CP [12-14].
In the current study we examine the cardiac autonomic state at rest, in response to a submaximal aerobic test and recovery from the test in children with CP and age matched TD children.
Materials and Methods
Sample size
The sample size calculation was based on the assumption that children with CP present lower HRV values at rest compared to TD children. Based on the study conducted by Kholod [6], where a large effect size was noted in HRV at rest between children with CP and TD children (type one error of 0.05 and 80% power), the sample size needed was at least 20 children in each group.
Participants
Twenty-five children aged 6-11 years, GMFCS I-III from the CP outpatient clinic at XXX Hospital in XXXX, and a convenient sample of 20 TD children, age and gender match as control group, participated in the study. Children with cardiovascular and/ or respiratory disease were excluded. The study protocol was approved by the hospital’s ethics committee and by the ethics board of XXXX University. Informed consent was obtained from the parents prior to the study.
Tests and measurements
Anthropometric measurements: body weight and height measurements and body mass index (BMI) were calculated (kg/ m2) [15]. HRV was monitored by the Polar Advanced Heart Rate Monitor (RC800CX) [16]. An elastic electrode transmitter belt (Polar sensor) was placed on the lower chest. The electrodes detect the voltage differential on the skin during every heart beat and send the signal wirelessly to the Polar receiver unit, which is then transferred via Polar-specific software (Polar® ProTrainer 5 software) to a computer. The data exported as a text file to the HRV analysis software (Kubios HRV software ver. 2.0; Biosignal Analysis and Medical Imaging Group, Department of Physics, University of Kuopio, Kuopio, Finland) for analysis of the following HRV parameters: Three measurements were calculated: the RR intervals, which are the differences between two successive R waves; the standard deviation of RR intervals (SDNN), which represents the total RR variability; and the square root of the mean squared of successive differences between adjacent RR intervals (RMSSD), which represent the short term RR variability [7]. During rest, breathing frequency was recorded using the ProRelax software version 5.1 and chest belt by MINDLIFE Company. Exercise intensity was calculated with the Karvonen formula [17,18]; Target HR = [(max HR − resting HR) × %Intensity] + resting HR. The HR max was based on the recommendations in the study by Verschuren et al. [19].
Testing procedure
Children with CP attend the outpatient clinic for an annual follow-up visit. Each week, the first author (XX) screened the characteristics of all children intending to come and noted those who met the basic inclusion criteria. The first child arriving at the clinic who met the criteria participated in the study. The testing procedure took about one hour. The same procedure was followed with the next child who met the basic criteria and so on with each child. The controls were children of hospital workers who agreed to their participation in the study.
After fastening the polar belt to the chest, the child sat quietly at rest for 5 minutes, and then walked on the treadmill using the Modified Naughton treadmill protocol [20]. Each stage lasted 2 minutes. The test ended when HR reached the value of the predicted maximal HR for 80% intensity of effort based on the Karvonen formula, or when the child could no longer walk at that speed level and asked to stop the test [17-19]. This was followed by five minutes of rest while sitting, after which the chest belt was removed and the child was free to leave.
Statistical analysis
Descriptive statistics summarized participant characteristics; differences between groups were presented by t-test or χ2 as appropriate. The Kaplan-Meier product-limit survival curve graphically summarized test stage survival rates in each group at each test stage. The Cox proportional hazards regression model was used. A repeated measure ANOVA was used to assess RR, SDNN, and RMSSD differences, with within subject factor (the pre-test rest stage, test last stage, and recovery post-test stage) and group as between subjects’ factor (TD vs. CP). Statistical significance was set at p ≤ 0.05. Statistical analyses were done using the Statistical Package for Social Sciences (SPSS version 22) (SPSS Inc., Chicago, IL, USA).
Results
No significant differences were noted in age, gender, and BMI values between groups. Fourteen of the children with CP had functional pattern diplegia, and 11 had hemiplegia (Table 1).
| TD N=20 | CP N=25 | p-value | |
|---|---|---|---|
| Male Female |
14 (70.0) 6 (30.0) |
17 (70.3) 8 (29.7) |
0.8 8 |
| Age (years) | 9 .0 ± 1.9 (6-11) |
9.4 ± 2.0 (6-11) |
0.44 |
| BMI (kg/m2) | 16.1 ± 2.1 (13.3-22.6) |
16.1 ± 3.1 (12.7-24.8) |
0.99 |
| Diplegia Hemiplegia |
1 4 (59.2) 11 (40.8) |
Values are frequency (percentage), Mean ±SD, Median [Min-Max). p-value is based on χ2, t-test.
Table 1: Participant characteristics by group.
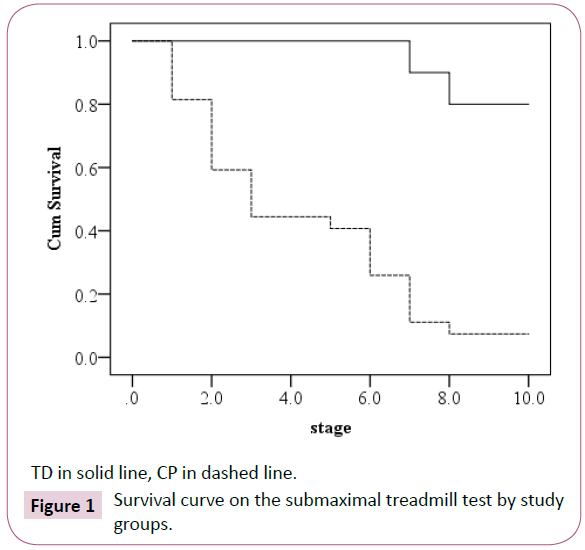
The survival curve for the test stage in each study group is presented in Figure 1. The median submaximal treadmill test stage was 3 for children with CP (95% CI 1.75-4.25). Sixteen of the twenty TD children completed all test stages (median test stage is 10). The Log Rank statistics (χ2 1=28.02) was highly significant (p<0.0001). The Cox regression model revealed that the odds ratio of completing the test was 11.2 times greater in TD children compared to children with CP. The intensity level based on the Korvanon formula differed significantly between children with CP and TD children (0.47 ± 0.16 vs. 0.64 ± 0.22 respectively, t43=2.65, p=0.01).
All HRV mean values at rest were significantly lower among children with CP. In addition, a significant interaction effect was found in all measures. In children with CP there was a smaller reduction in RR values during the test, and a smaller increase in mean RR value during the recovery period compared to TD children (interaction effect F2:86=5, p=0.008) (Table 2). A larger reduction in SDNN values during the test was noted among TD children compared to children with CP (interaction effect F2:86=11, p<0.001) (Table 2). The same trend was found for the RMSSD values; a larger reduction in RMSSD values during the test and a larger increase in the mean RMSSD values during the recovery period was noted in TD children (interaction effect F2:86=5, p<0.01) (Table 2).
| Study Phase | F(d.f)/*p-value | F(d.f) / *p-value | |||||
|---|---|---|---|---|---|---|---|
| Rest | Test | Recovery | Within each group | Between groups | Within groups | Interaction | |
| RR | |||||||
| TD | 725 ± 128 | 373 ± 81 | 470 ± 66 | F2:38=39, p<0.01 | F1; 43=0.26 0.61 |
F2; 86=155 <0.01 |
F2; 86=5 <0.01 |
| CP | 655 ± 85 | 413 ± 66 | 478 ± 65 | F2;48=28, p<0.01 | |||
| t43/ ^p-value | 2.18/0.03 | ||||||
| SDNN | |||||||
| TD | 80 ± 33 | 12 ± 5 | 69 ± 26 | F2:38=65, p<0.01 | F1; 43=5 0.01 |
F2; 86=80 <0.01 |
F2; 86=11 <0.01 |
| CP | 52 ± 17 | 24 ± 13 | 56 ± 25 | F2;48=26, p<0.01 | |||
| t43/ ^p-value | 3.35/<0.01 | ||||||
| RMSSD | |||||||
| TD | 41 ± 16 | 5 ± 3 | 20 ± 17 | F2:38=31, p<0.01 | F1; 43=3 0.12 |
F2; 86=72 <0.01 |
F2; 86=5 <0.01 |
| CP | 29 ± 13 | 7 ± 3 | 19 ± 12 | F2;48=30, p<0.01 | |||
| t43/ ^p-value | 2.77/<0.01 | ||||||
^p-value- between groups at rest based on independent t-test.
*p-value- based on repeated measures ANOVA.
Table 2: Mean and SD of HRV parameters in milliseconds at each study phase by group.
Discussion
The main findings of the current study are that HRV values at rest are significantly lower, and during the submaximal test, responsive but less among children with CP compared to TD children. Performing a submaximal aerobic test presented significant differences between groups, where children with CP “survived” in the test significantly less.
Hyper function of the sympathetic branch of the cardiac autonomic system at rest in children with CP has been presented in previous studies [6,21-24], and the findings of the current study strengthen those results. The significantly lower autonomic response to the submaximal treadmill test in children with CP is in line with findings of previous studies regarding the cardiac autonomic response to different stimuli. In those studies, different manipulations were used [9-12]. The cardiac autonomic response to passive standing [12] or to the 6-minute walk test [9] was significantly lower in children with CP as compared to TD children.
One of the most interesting questions raised is whether the observed autonomic response to activity, is a sign for therapeutic intervention. The autonomic impairment is due to brain insult or to more sedentary behavior, or perhaps both. Because the current study is cross sectional it can only provide associations. Nevertheless, its results may highlight the potential of children with CP to activate this system by physical activity, and may be by that to have a more balanced cardiac autonomic response to activity. In healthy children [21] and adults [9], as well as in people with disabilities [22], performing aerobic exercise is associated with a reduced HR and an increased HRV at rest. In the current study, the HRV values at rest of children with CP who performed physical activity regularly were not significantly different compared to children with CP who were not active. This might lead to two different assumptions. The first is that the physical activity was not intense enough to cause an effect on the cardiac autonomic system of children with CP. This assumption is strengthened by the improvement in HRV in response to physical activity seen in children with CP who performed very intense, short duration exercises [25]. It might be that in order to have an impact on the cardiac autonomic system in children with CP vigorous exercise programs are needed, different than those sufficient for TD children.
On the other hand, the response to the sub-maximal test of the HRV parameters of those children with CP who participated on a regular basis in some form of physical activity was not significantly different than that of TD children. In addition, no differences in functional pattern or mobility were noted between those who were physically active and those who were not. There is an assumption that physical activity, performed regularly for at least four months, contributes to more balanced autonomic performance during the test, thus strengthening the assumption that, at least in part, the autonomic impairment in children with CP is due to sedentary behavior.
The small sample for the sub-group analysis is the primary limitation of the current study. In addition, being active was defined based on parental statements and not on direct assessment of the child’s daily performance, which may generate some bias. The current study is cross sectional in its data collection method. One can argue that those children with more reactive autonomic systems were those who tolerated performing physical activity at baseline and therefore could regularly perform physical exercise.
Conclusion
The results of the current study provide a rationale for further studies of this topic. Children with CP show a lower HRV at rest, and less adaptive to exercise as compared to TD children. Performing physical activity is highly recommended for children with CP. Cardiac autonomic response to exercise is an essential component in achieving the desirable treatment effect; re-educating the cardiac autonomic system. Assessing the autonomic response to different exercise protocols is the next step needed.
References
- Blair E, Stanley FJ (1997) Issues in the classification and epidemiology of cerebral palsy. Ment Retard Dev Disabil Res Rev 3: 184-193.
- Peterson MD, Gordon PM, Hurvitz EA (2013) Chronic disease risk among adults with cerebral palsy: the role of premature sarcopoenia, obesity and sedentary behaviour. Obes Rev 14: 171-182.
- Amichai T, Katz-Leurer M (2014) Heart rate variability in children with cerebral palsy: review of the literature and meta-analysis. Neuro Rehabilitation 35: 113-122.
- Plasschaert F, Jones K, Forward M (2011) The clinical relevance of selecting resting data at different points in an energy cost of walking test in cerebral palsy. Dev Med Child Neurol 53: 245-249.
- Fleming S, Thompson M, Stevens R, Heneghan C, Plüddemann A, et al. (2011) Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 377: 1011-1018.
- Kholod H, Jamil A, Katz-Leurer M (2013) The associations between motor ability, walking activity and heart rate and heart rate variability parameters among children with cerebral palsy and typically developed controls. Neurorehabilitation 33: 113-119.
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J 17: 354-381.
- Samuels MA (2007) The brain–heart connection. Circulation 116: 77-84.
- Iwasaki KI, Zhang R, Zuckerman JH, Levine BD (2003) Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J Appl Physiol 95: 1575-1583.
- Grant CC, Viljoen M, Janse van Rensburg DC, Wood PS (2012) Heart rate variability assessment of the effect of physical training on autonomic cardiac control. Ann Noninvasive Electrocardiol 17: 219-229.
- Thayer JF, Yamamoto SS, Brosschot JF (2010) The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 141: 122-131.
- Murphy NA, Carbone PS (2008) Promoting the participation of children with disabilities in sports, recreation, and physical activities. Pediatrics 121: 1057-1061.
- Damiano DL (2006) Activity, activity, activity: rethinking our physical therapy approach to cerebral palsy. Phys Ther 86: 1534-1540.
- Novak I, Mcintyre S, Morgan C, Campbell L, Dark L, et al. (2013) A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol 55: 885-910.
- Day SM, Strauss DJ, Vachon PJ, Rosenbloom L, Shavelle RM, et al. (2007) Growth patterns in a population of children and adolescents with cerebral palsy. Dev Med Child Neurol 49: 167-171.
- Gamelin FX, Berthoin S, Bosquet L (2006) Validity of the polar S810 heart rate monitor to measure R-R intervals at rest. Med Sci Sports Exerc 38: 887-893.
- American College of Sport Medicine (1986) Guidelines for exercise testing and prescription (3rd Edn). Philadelphia: Lea & Febiger Pp: 18-19.
- Karvonen MJ, Kentala E, Mustala O (1957) The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn 35: 307-315.
- Verschuren O, Maltais DB, Takken T (2011) The 220-age equation does not predict maximum heart rate in children and adolescents. Dev Med Child Neurol 53: 861-864.
- Goldberg L, Elliot DL, Kuehl KS (1988) Assessment of exercise intensity formulas by use of ventilatory threshold. Chest 94: 95-98.
- Nagai N, Hamada T, Kimura T, Moritani T (2004) Moderate physical exercise increases cardiac autonomic nervous system activity in children with low heart rate variability. Childs Nerv Syst 20: 209-214.
- Giagkoudaki F, Dimitros E, Kouidi E, Deligiannis A (2010) Effects of exercise training on heart-rate-variability indices in individuals with Down syndrome. J Sport Rehabil 19: 173-183.
- Yang TF, Chan RC, Kao CL, Chiu JW, Liu TJ, et al. (2002) Power spectrum analysis of heart rate variability for cerebral palsy patients. Am J Phys Med Rehabil 81: 350-354.
- Park ES, Park CI, Cho SR, Lee JW, Kim EJ (2002) Assessment of autonomic nervous system with analysis of heart rate variability in children with spastic cerebral palsy. Yonsei Med J 43: 65-72.
- Cohen-Holzer M, Sorek G, Kerem J, Schless S, Freedman R, et al. (2016) The influence of intense combined training on upper extremity function in children with unilateral cerebral palsy: does initial ability matter? Phys Occup Ther Pediatr 36: 376-387.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences