Meta-Analysis Showing Improvement in GMFM and PEDI Scores after Allogeneic Stem Cell Transplantation for Cerebral Palsy
Sabiha Shamim1,4, David Lawrence Greene1,2,3,4, Umm E Habiba1,4, Khalil Ahmad5, Nasar Khan1,2,3,4* and Amna Umer1,4
1R3 Medical Research LLC, Scottsdale, United States 2Bello Bio LLC, Scottsdale, United States 3Bello Bio Labs and Therapeutics Pvt. Ltd., Islamabad, Pakistan 4Pak-American Hospital, Islamabad, Pakistan 5Department of Statistics, Quaid-I-Azam University, Islamabad, Pakistan
Published Date: 2023-08-16Sabiha Shamim1,4, David Lawrence Greene1,2,3,4, Umm E Habiba1,4, Khalil Ahmad5, Nasar Khan1,2,3,4* and Amna Umer1,4
1R3 Medical Research LLC, Scottsdale, United States
2Bello Bio LLC, Scottsdale, United States
3Bello Bio Labs and Therapeutics Pvt. Ltd., Islamabad, Pakistan
4Pak-American Hospital, Islamabad, Pakistan
5Department of Statistics, Quaid-I-Azam University, Islamabad, Pakistan
- *Corresponding Author:
- Nasar Khan
Pak-American Hospital, Islamabad,
Pakistan,
Email: nkhan@r3stemcell.com
Received date: July 17, 2023, Manuscript No. IPCDD-23-17249; Editor assigned date: July 19, 2023, PreQC No. IPCDD-23-17249 (PQ); Reviewed date: August 02, 2023, QC No. IPCDD-23-17249; Revised date: August 09, 2023, Manuscript No. IPCDD-23-17249 (R); Published date: August 16, 2023, DOI: 10.35841/2471-1786.9.3.78
Citation: Shamim S, Greene DL, Habiba UE, Ahmad K, Khan N, et al. (2023) Meta-Analysis Showing Improvement in GMFM and PEDI Scores after Allogeneic Stem Cell Transplantation for Cerebral Palsy. J Child Dev Disord Vol.9 No. 3: 78.
Abstract
Cerebral Palsy (CP) is a permanent condition indicated by a host of complications that affect the neurologic system and is usually diagnosed based on motor and nutritional function, cognizance and social ability. This analysis considered outcome measure results for GMFM-66, GMFM-88 and PEDI from baseline to their 3-month, 6- month, 12-month and 24-month follow-ups. According to the combined estimates of the follow-ups, the scores of GMFM-66 and GMFM-88 of the groups treated with SCT showed an increase from the baseline standardized mean difference respectively. The score for PEDI in groups treated with SCT significantly dropped from the baseline. Transplantation of stem cells has beneficial effects on cerebral palsy.
Keywords
Stem cell therapy; Cerebral palsy; Allogeneic; GMFM; PEDI; Cellular
Introduction
Cerebral Palsy (CP) is a permanent neurodevelopmental disorder affecting multiple functions of the body, including but not restricted to movement, communication, nutrition, understanding and judgment [1,2]. Eggenberger, et al., cite premature births and asphyxiation during the gestation/delivery of a baby as common complication that leads to brain damage which may result in cerebral palsy. Multiple factors, such as perinatal stroke, truncated infant weight, complications during birth, gestational age, multiple births and infection, are considered contributors to the development of cerebral palsy [3]. The most prevalent physical handicap brought on by harm to the still-developing brain in children is cerebral palsy. This complicated combination of motor symptoms ranges from moderate motor coordination failure to substantial quadriplegia or hemiplegia [4]. Cerebral palsy is usually diagnosed based on motor function and postural issues that may appear as early as during the 1st year of the child and stay lifelong; these issues are non-progressive but do alter with age [5]. These motor functions can be evaluated using different measures.
The Gross Motor Function Measure (GMFM) is the most often used scale for evaluating motor function in CP patients aged five months onwards. The 88-question scale has been modified to 66 questions. Both variants are frequently used; however, the 88- questionnaire has been evaluated for other neurologic disorders, such as Down syndrome [6]. Both GMFM outcome measures evaluate lying and rolling, sitting, crawling and kneeling, standing and walking and running and jumping. The grading is based on the ability of the patient to carry out the tasks rather than the quality of the task performed. The scores are calculated as a percentage ranging from 0 to 100, with a higher score denoting greater ability. The Pediatric Evaluation of Disability Inventory (PEDI) is another outcome measure used to evaluate CP patients. This scale assesses self-care, mobility and social function by testing functional capabilities and performance in children with disabilities. Higher PEDI scores indicate better ability [7]. For each of the six categories in PEDI, the "Minimally Important Difference" (MID) is calculated based on physician reports. The MID ranges from 6.0 to 15.6 units and a score change of about 11 units has been recommended as a significant clinical difference [8].
A recent study was conducted to find the prevalence of CP; the researchers noticed a dearth of data from lower- and middle-income countries [9]. However, they noticed that the overall birth prevalence (1.6 per 1000 live births) was twentyfive percent lower from 2013 (2.1 per 1000) for the high-income countries. Data from only six low-income countries could be considered. Still, the results showed that the prevalence in these countries, such as Bangladesh, was considerably higher (more than 3 per 1000 children). Researchers from Europe found in a study that more than forty percent of children with CP had white matter impairment [10]. The MRI also revealed basal ganglia lesions, cortical/subcortical lesions, abnormalities, localized infarcts and other lesions. The way that CP is treated has developed over time. Traditional rehabilitation techniques, including physical therapy, occupational therapy, speech therapy, assistive technology, pharmaceutical intervention and surgery like rhizotomy and neurectomy, are ineffective for treating cerebral palsy.
The safety, efficacy and mechanism of action of stem cell transplantation in preclinical trials have previously been conducted [11]. Recent research suggests that stem cell therapy may be a successful treatment for CP because of the capacity of stem cells for multidirectional differentiation and movement. Different important molecules secreted by stem cells, along with mechanisms including but not limited to angio-and neurogenesis, neuroplasticity and immunomodulation, have been proposed as factors contributing to positive results [12]. Clinical attention has switched to using adult stem cells to alleviate ethical or safety concerns. Adipose tissue, Bone Marrow (BM), or stem cells generated from the umbilical cord are the three main adult stem cell sources for treating CP [13]. As cited by [14], applying autologous cellular therapy would be a better option; however, most CP patients don't have their umbilical cords preserved. Hence, alternate sources of cells must be explored.
Currently, seven clinical trials are registered with clinicaltrials.gov under the categories of allogeneic stem cells and cerebral palsy, out of which three are still recruiting (Checked: 24 January 2023; Rechecked: 26 May 2023). A systematic review and meta-analysis were previously conducted to explore the tolerability and efficacy of stem cell transplantation in people with cerebral palsy. The researchers used a good time frame (1990-2019), focusing on trials using only GMFM as an outcome measure. Even though GMFM is the most often used scale in patients with CP, further research must be conducted on other outcome measures. The studies were selected using PubMed and EMBASE databases only. Most of the articles present in EMBASE are also indexed in PubMed. Hence, adding another database could have been considered. A study that falls under the time frame selected under the meta-analysis by [1] had not been included, reasons for which are unclear [15]. This study has been included in the current meta-analysis. Another meta-analysis investigated randomized controlled trials for the safety and efficacy of Human MSCs for patients with CP [16]. The researchers screened articles from Embase, Cochrane Library, PubMed, Web of Science, Chinese Clinical Trial Registry and Clinical Trials.gov published till February 2020. Xie, et al., excluded non-randomized trials, case reports, cross-sectional studies and cohort studies. However, they were included in the current meta-analysis to get an overview of the efficacy of stem cell transplantation in CP patients. Our meta-analysis includes at least six studies [17-23] that have not been added in previously published meta-analyses (n=14).
Cellular therapy is emerging as an alternative treatment option. A simple literature search shows that there is a great interest in the application of stem cells for the alleviation of conditions (symptoms) of cerebral palsy. However, due to the complexity of this condition, different outcome measures have been used in most of the trials that have been previously conducted. Our meta-analysis aimed to investigate the effect of allogeneic stem cell treatment on motor function using GMFM-88, GMFM-66, and PEDI outcome measures in children with CP.
Materials and Methods
Selection criteria
The studies were included if: The research article had full-text availability in English; the study population consisted of children diagnosed with any type of CP; the treatment included any kind of allogeneic stem cell transplantation. The motor outcome of the study population was assessed and reported in the GMFM (GMFM-88 or GMFM-66) or PEDI. We included case studies, Randomized Controlled Trials (RCTs) and controlled trials, with or without blinded outcome assessment, that compared the outcomes of interventions with stem cells of any type versus standard care or no treatment at all or placebo (controls) in people with cerebral palsy. Studies were excluded if: The data was presented in languages other than English, the studies presented incomplete data or data was not present in the required form, or duplicates of the same publication.
Outcome measures
This analysis considered GMFM-66, GMFM-88 and PEDI from baseline to subsequent follow-ups.
Search strategy
The research articles were searched following the PRISMAstatement guidelines to perform a systematic electronic search using PubMed, Cochrane and Science Direct databases for trials published between 2010 and January 2023. We searched terms like "stem cells," "cellular therapy," and "cerebral palsy" in all the selected databases. Only human studies in English were considered. Potential research articles were identified through the title, keyword and abstract screening and were further screened according to their content, keeping the inclusion and exclusion criteria in consideration see Table 1.
| Reference | Country | Method | Participants | Study groups | Cell type | Cell dose | Cell source | Transplant method | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Chen, et al., 2010* [15] | China | RCT. N=33 | Age: 1-12 years old. Any type of confirmed CP | Treatment group n=18: OEC and non-altered physiotherapy. Control group n=15 | OEC | 2 × 106 cells | Human fetal olfactory bulb | Stereotactic method | 6 |
| Luan, et al., 2012* [24] | China | RCT. n=94 | Age: 0-4 years with severe CP | Treatment group n=45: NPC and rehabilitation therapy. Control group n=49: Only rehabilitation therapy | NPC | 8-10 × 106 cells | Fetal forebrain tissue | Injection into the fontanelle | 6, 12 |
| Min, et al., 2013* [14] | South Korea | RCT, placebo, double-blind. N=105 | Age: 10 months-10 years with CP | Treatment group n=31: pUCB, EPO (n=33). Control (n=32) | UCB, rhEPO | UCB was minimum 3 × 107/kg TNC. Two rhEPO injections at a dose of 500 IU/kg | UCB | IV | 1,3 and 6 |
| Kang, et al., 2015* [25] | South Korea | RCT, placebo, double-blind trial. N=36 | Age: 6 months-20 years old with CP | UCB group n=18. Control group n=18 | UCB | 1.0-7.10 × 107 TNC/kg | CB | IV or IA | 1,3 and 6 |
| Huang, et al., 2018* [26] | China | RCT, placebo. N=54 | Age: Between 3-12 years with any type of CP | hUCB-MSC group n=27: SCT with basic rehabilitation. Control group n=27: patients received normal saline (0.9% NS) with basic rehabilitation | hUCB-MSCs | 4 infusions of hUCB-MSCs fixed dose of 5 x 107 | CB | IV | 3,6,12 and 24 |
| Fu, et al., 2019* [27] | China | Retrospective study. N=57 | Age: 1 month to 12 years with spastic CP | One course group (n=30), double course group (n=27) | hWJSC | 4 or 8 × 107 hWJSCs | Human Wharton’s Jelly | IT | 6, 12 |
| Gu, et al., 2020 [28] | China | Parallel, double-blinded, placebo RCT. N=39 | CP patients aged 2-12 years were included | Treatment group: four transfusions of hUC-MSCs along with rehabilitation. Control group: Placebo along with rehabilitation | Human UCMSCs | 4 transfusions. (Cell count: 4.5~5.5 × 107) | Wharton's Jelly | IV | 1, 3, 6, and 12 |
| Min, et al., 2020* [20] | South Korea | 2 × 2 factorial (four arm) RCT placebo. N=92 | Age: 10 months-6 years with CP | (A) UCB+EPO, (B) UCB+placebo EPO, (C) placebo UCB+EPO and (D) placebo UCB+ placebo EPO | UCB and erythropoietin | Total Nucleated Cell (TNC) number of ≥ 3 × 107/kg UCB, and 500 IU/kg human recombinant EPO (six times) | Cord blood | IV | 1, 3, 6, and 12 |
| Amanat, et al., 2021* [17] | Iran | Randomized double-blind sham-controlled clinical trial. N=36 | Spastic CP patients aged: 4-14 years. Only CP patients with GMFCS level 2-5 and white matter lesions in brain MRI were included | UCT-MSC: n=36. Control: n=36. Rehabilitation | UCT | Treatment group: Single-dose (2 × 107) cells. Needle pricks to the lower back were performed in the sham-control arm | UCT | IT | 1, 3, 6 and 12 |
| Sun, et al., 2021* [21] | US | Phase 1, RCT. N=15 | Age: 2-4 years. Children with hypertonic CP | All individuals received stem cells. | hCT-MSC OR UCB | A single dose of ≥ 2.5 × 107 cells/kg | UCT or UCB | IV | 6, 12, and 24 |
| Hu, et al., 2022* [18] | China | N=5 | 4-10 years old | All individuals received stem cells. | ADMSC | (107 ADMSCs/kg). One course of treatment was defined as 3 consecutive infusions. Each infusion dose was determined according to the body weight of the participant. | Periumbilical region of the participants’ mothers | IV | 6 and 12 |
| Lv, et al., 2022* [19] | China | Open-label RCT. N=25 | CP patients aged 3-12 years. Moderate to severe paralysis characterized by spastic CP induced by ischemic hypoxia | Treatment group (n=15) NSCs and rehabilitation therapy. Control group (n=10), received rehabilitation therapy | NSCs | NSCs (5 × 105/kg) | Aborted human fetal forebrain tissue | IN | 1, 3, 6 and 24 |
| Sun, et al., 2022* [22] | US | Phase I, open-label study. N=15 | Age: 1-6 years. Children with moderate to severe spastic CP | Allogenic cord blood group (n=20). hCT MSC group (n=23). Natural history (n=25) | CB | Cell dose of ≥ 2.5 × 107 cells/kg based on the pre-cryopreservation count (median infused cell dose, 3.3 x 107; range, 1.8-5.2 × 107) | CB | IV | 3, 6, 12, 24 |
| Zarrabi, et al., 2022* [23] | Iran | A multi-center, randomized, double-blind, population-based clinical study with sham-control group | Ages: 4 to 14 years old with spastic CP | UCB MNC group (n=36), Sham group (n=36) | UCBMNC | A single dose of 5 × 106 /kg | CB | IT | 1, 3, 6, and 12 |
Table 1: Summary of characteristics of included studies.
Data extraction and study characteristics
Two reviewers (Shamim S and Habiba UE) screened the fulltext content of scholarly articles on cellular therapy in CP and extracted data. The gathered data was reviewed by a third independent investigator (Khan N). Relevant features of the selected studies were summarized in a Table 1. These included country of origin, population size, range of age of patients, intervention (stem cell type, dose, route of administration) and available follow-ups. The extracted data were analyzed (Ahmed K).
Statistical data analysis
This meta-analysis used the Weighted Mean Difference (WMD) and the Standardized Mean Difference (SMD=Baseline- Stem Cell-treated group) to compare continuous variables between study groups. P-values less than 0.05 and Confidence Intervals (CI) of 95% were considered statistically significant. The I2 statistic was used to determine the heterogeneity of the included studies; I2 values of 25%, 50%, and 75%-100% indicated low, medium and high heterogeneity in the included research, respectively. When the effects were determined to be diverse (I2%>50% and P<0.10), we employed a random-effects model for the meta-analysis [29]; otherwise, the data were analyzed using a fixed-effects model. In this meta-analysis, we compared the treatment group, i.e. Stem cells, and the control group (if any) from the included studies using Jamovi version 2.3 [30] and displayed the results on forest plots (Figures 1-3). In this investigation, heterogeneity and risk of bias were evaluated using the Cochrane Q test and I2 statistic, evaluating the methodological quality using the Cochrane ROB and metaregression analysis and assessing publication bias with a funnel plot, Begg's and Egger's regression tests. In addition, study design, participant age, and the time interval between diagnosis and intervention, which may influence heterogeneity, were considered.
Results
Search results
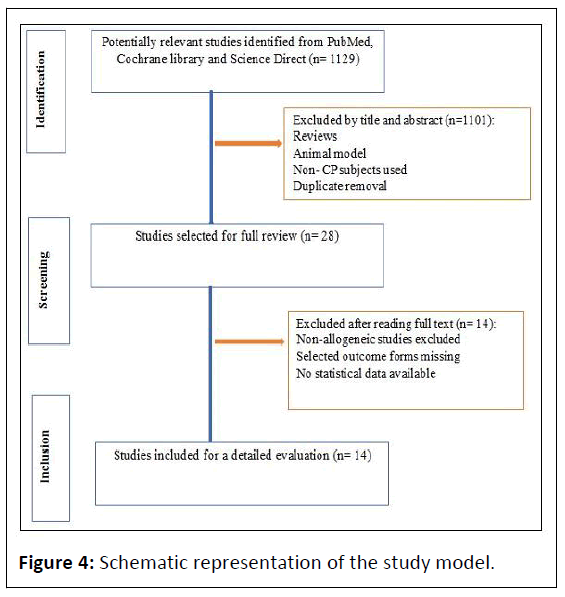
The search strategy identified 1129 articles from PubMed, Cochrane and Science Direct. After reviewing the titles and abstracts, 1101 articles were excluded as they were either not reporting any new study and just reviewed, or they were focused on preclinical data with animal models, had subjects who were not cerebral palsy patients, or were replicates of the articles that had already been considered. Twenty-eight articles were screened for a full review. After thoroughly examining studies in which autologous cells were transplanted, GMFM-66/GMFM-88/ PEDI was not used, or the unavailable statistical data were excluded. Finally, fourteen articles were finalized and included in this meta-analysis. The study selection process is illustrated in Figure 4.
Characteristics of the included studies
The fourteen allogeneic studies included were conducted in different countries; eight were from China, three were from South Korea, and two were from Iran and the US. Eleven studies were randomized-controlled trials, and one was a retrospective study. Major characteristics of the included studies have been outlined (Table 1). The studies used a variety of sources for cellular therapy. Two studies used a combination of Umbilical Cord Blood (UCB) and human erythropoietin; two studies used umbilical cord blood; two studies used Umbilical Cord MSCs (UCMSC); a study each was conducted using Olfactory Ensheathing Cells (OEC), Neural Progenitor Cells (NPC), human Wharton's Jelly Stem Cells (hWJMSC), Umbilical Cord Tissue MSCs (UCT-MSCs), Adipose-Derived MSCs (ADMSC), Neural Stem Cell (NSC), Umbilical Corsd Blood Mononuclear Cells (UCB-MNC) and a combination of either UCT or UCB. Various routes of administration were employed, OECs were given stereotactically, NPCs were injected into the fontanelle, seven studies used intravenous administration, and three studies used intrathecal administration. The dosage of cellular therapy given to the patients varied greatly in each study. Some studies recorded a fixed number of cells administered [ranging from 2 x 106 cells to 4 (5 x 107 cells)]; some studies speci ied total nucleated cells administered by weight of the subjects (ranging from 1.0 x 107 TNC/Kg to 10 x 107 TNC/Kg) (Table 1).
The ages of the participants ranged from 0-14 years. All participants included had been diagnosed with cerebral palsy. The studies recorded different follow-up times; nine studies recorded 3-month follow-ups, all studies recorded 6-month follow-ups, ten studies recorded 12-month follow-ups and four studies recorded 24-month follow-ups (Table 1). The initial metaanalysis search showed that CP patients were observed using different assessment types that included structured questionnaires [e.g., Modi ied Ashworth scale and CP-Quality of Life [17]], brain scans [e.g., MRI [26]] and manual observations recorded by researchers and guardians of the patients including qualitative assessments using Likert scale [31]. However, only studies with either GMFM-88, GMFM-66, or PEDI as outcome measures were considered for this analysis.
Effects of stem cell therapy on GMFM-66 of patients with cerebral palsy
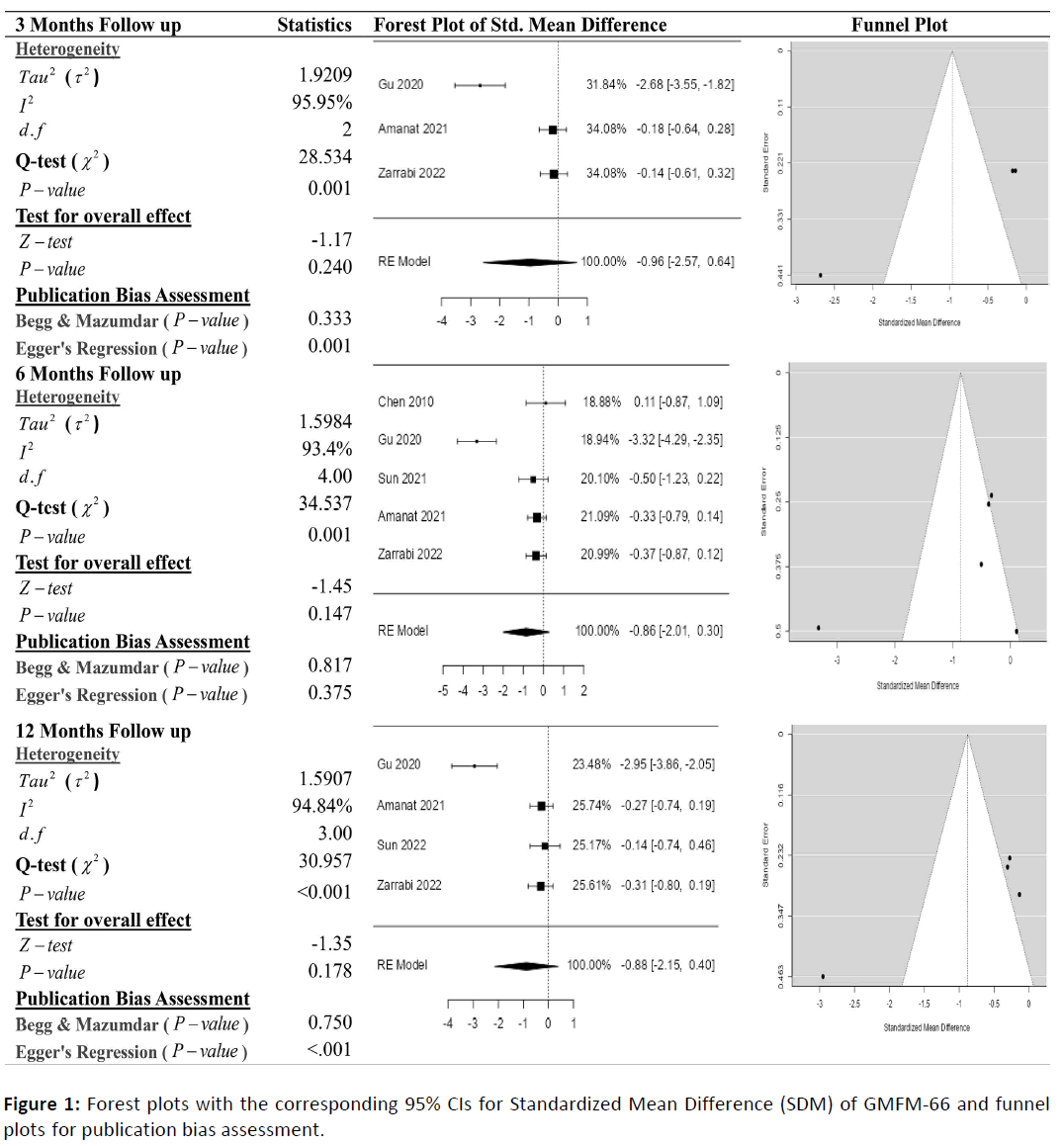
Figure 1 shows the meta-analysis results for the score of GMFM-66 with 3-, 6-, and 12-month follow-ups, demonstrating that stem cell transplantation is associated with improvement of GMFM-66 scores. It was found through meta-analysis the SMD of GMFM-66 was statistically non-significant for cerebral palsy with 3-, 6-, and 12-month follow-ups. According to the forest plots, the overall effect size measured with SMD revealed comparing the administration of the stem cells and control group, which had shown the increment in the score of GMFM-66 at 5% level of significance in 3-, 6-, and 12-months follow-up as (SMD: -0.96, 95% CI: -2.57 to 0.64, P-value: 0.240>0.05, I2: 95.95%) and (SMD: -0.86, 95% CI: -2.01 to 0.30, P-value: 0.147>0.05, I2:93.4%), and (SMD: -0.88, 95% CI: -2.15 to 0.40, P-value: 0.178>0.05, I2: 94.84%) respectively. The SMDs in all follow-ups were statistically non-significant. Still, the observed SMDs in all included studies, along with 3-, 6-, and 12- month follow-ups, were found to be negative (91.67%), which indicated that the increase in the score of GMFM-66 was due to stem cell transplantation.
Effects of stem cell therapy on GMFM-88 of patients with cerebral palsy
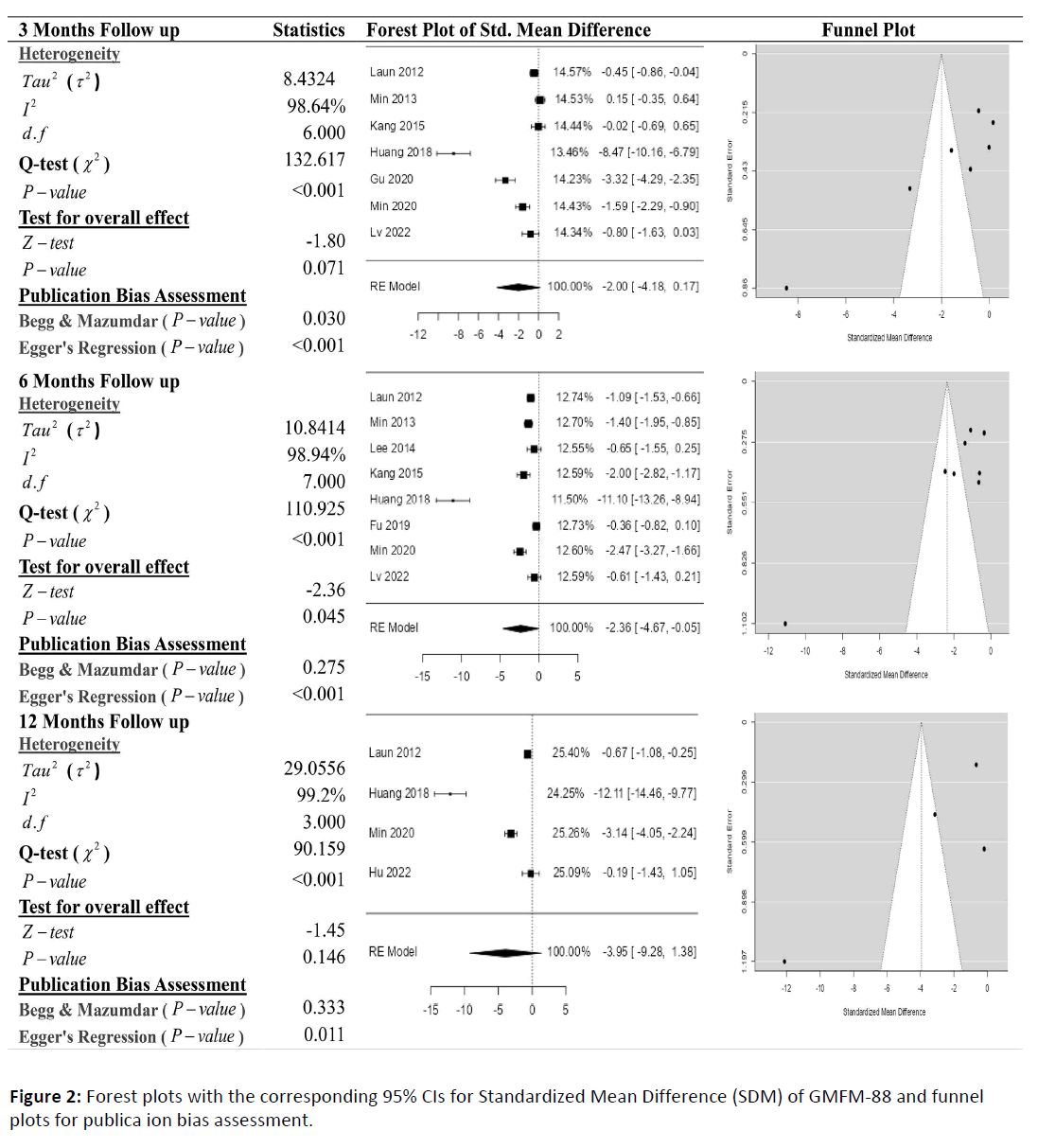
Figure 2 represent the results of the meta-analysis for the score of GMFM-88 with 3-, 6-, 12, and 24-month follow-up demonstrating that stem cell transplantation is associated with improvement of GMFM-88 scores. According to the forest plots, the overall effect size measured with SMD revealed comparing the administration of the stem cells and control group, which had shown the increment in GMFM-88 at a 5% level of significance in 3- 6-, 12- and 24-months follow-up as (SMD: -2.00, 95% CI: -4.18 to 0.17, P-value: 0.071>0.05, I2: 98.64%),(SMD: -2.36, 95% CI: -4.67 to -0.05, P-value: 0.045<0.05, I2: 98.94%), and (SMD: -3.95, 95% CI: -9.28 to 1.38, P-value: 0.146>0.05, I2: 99.20%) respectively. The SMDs in all follow-ups were statistically non-significant. Still, the observed SMDs in all included studies, along with 3-, 6-, 12- and 24-month followups, were negative (100%), indicating the increase in the score of GMFM-88 due to stem cell transplantation.
Effects of stem cell therapy on PEDI of patients with cerebral palsy
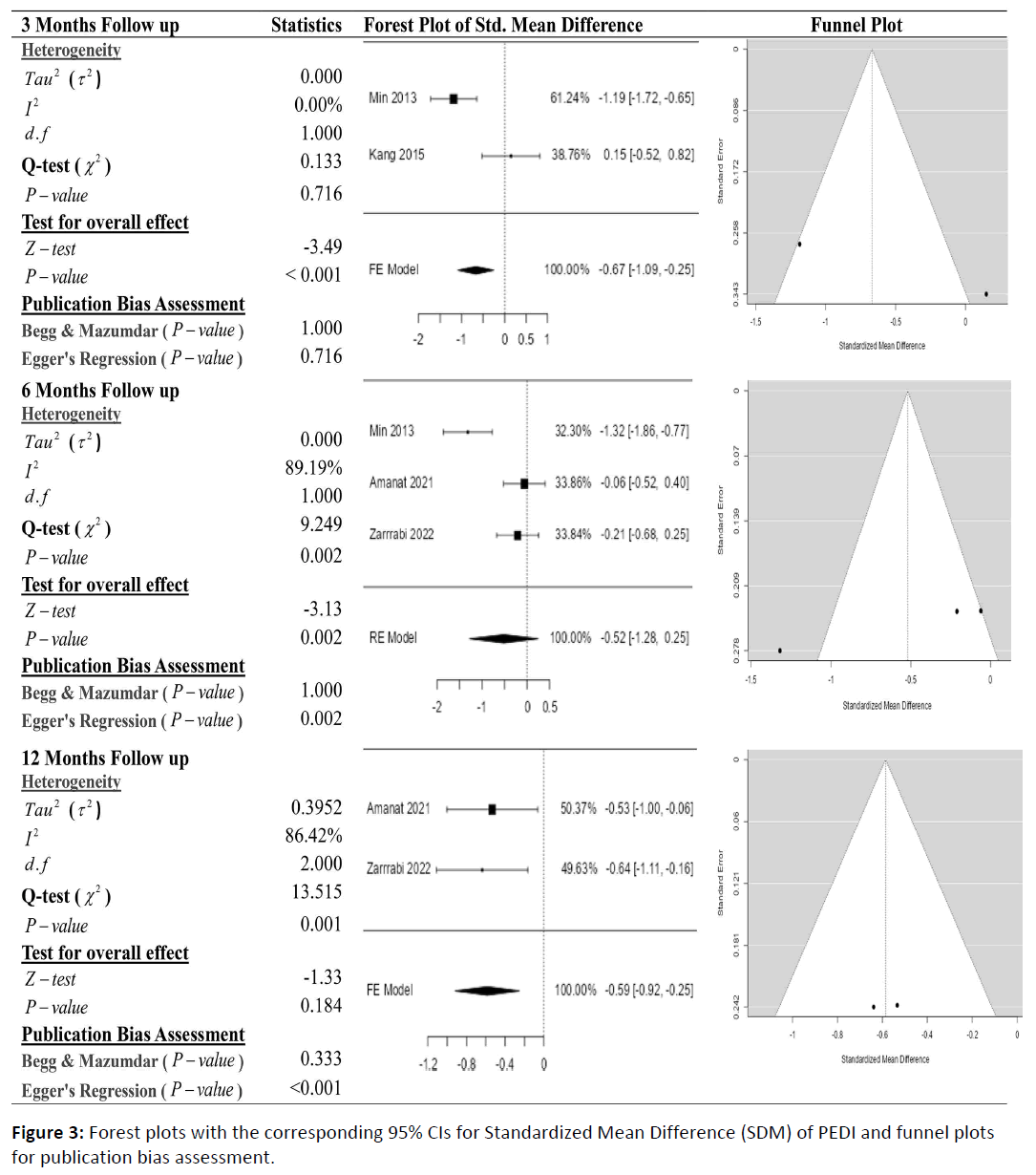
Figures 3 shows the results of the meta-analysis for the PEDI scores with 3-, 6-, and 12-month follow-ups, demonstrating that stem cell transplantation is associated with improvement in the score of PEDI. According to the forest plots, the overall effect size measured with SMD revealed comparing the administration of the stem cells and control group, which had shown a significant increment in PEDI scores at a 5% level of significance in 3-and 6-months follow-up as (SMD: -0.67, 95% CI: -1.09 to -0.25, P-value: 0.716>0.05, I2: 0%) and (SMD: -0.52, 95% CI: -1.28 to 0.25, P-value: 0.002<0.05, I2: 89.19%) respectively. But the overall effect size measured revealed comparing the administration of the stem cells and control group, which had shown the non-significant increment in PEDI scores at a 5% level of significance in 12-month follow-up as (SMD: -0.59, 95% CI: -0.92 to -0.25, P-value: 0.001<0.05, I2: 86.42%). The observed SMDs in all included studies along with 3-, 6-, and 12-months follow-up were found to be negative (85.71%) which indicated that the increase in PEDI scores was due to stem cells transplantation.
Heterogeneity
Cochran's Q-test and I2 statistic was applied to measure the heterogeneity of the true scores of the parameters: GMFM-66, GMFM-88 and PEDI with 3-, 6-, 12- and 24-month follow-up. According to the Q-test, the actual outcomes appeared to be heterogeneous significantly for the score of GMFM-66 with 3-, 6-, and 12-month follow-up as (Q-test: 28.534, P-value: 0.001<0.05, tau2: 1.9209, I2: 95.95%), (Q-test: 34.537, P-value: 0.001<0.05, tau2: 1.5989, I2: 93.4%) and (Q-test: 30.957, P-value: 0.001<0.05, tau2: 1.5907, I2: 94.84%). Similar results can be found in Figures 3 and 4, where the random effect model is implemented for significant heterogeneous true outcomes; otherwise, the fixed effect model was used.
Risk of bias assessment
The assessment of the risk of bias is estimated through funnel plots, Begg's, and Egger's regression tests for each forest plot of the scores of the parameters: GMFM-66, GMFM-88, and PEDI with 3-, 6-, and 12-month follow-up. The publication bias analysis indicated a non-significant bias at a 5% level of significance for GMFM-66 in all 3-, 6-, and 12-month follow-ups as (Begg & Mazumdar test, P-value: 0.233>0.05 and Egger's regression P-value: 0.135>0.05), (Begg & Mazumdar test, Pvalue: 1.000>0.05 and Egger's regression P-value: 0.001<0.05), (Begg & Mazumdar test, P-value: 0.233>0.05 and Egger's regression P-value: 0.001<0.05), and (Begg & Mazumdar test, Pvalue: 0.333>0.05 and Egger's regression P-value: 0.057>0.05) respectively. Similar results for publication bias about the scores of GMFM-88 and PEDI with 3-, 6-, and 12-month follow-ups can be found in Figures 3 and 4.
Discussion
In this meta-analysis, we narrowed down 28 research articles from 1129, our initial search. Out of these 28, we finally included 14 studies for the meta-analysis with 717 patients (441 cellular therapy group, 276 control group). These studies combined case studies, retrospective studies and controlled and uncontrolled trials. This meta-analysis was conducted to get an overview of the efficacy of allogeneic stem cell transplantation on the gross motor function of CP patients using GMFM-66, GMFM-88, and PEDI scales. The statistical significance of their scores was measured as (P-value<0.05). The SMD of the three outcome measures (GMFM-66, GMFM-88, and PEDI) showed statistically non-significant but practical improvement in the treatment group from the control group (Figures 1-3). The SMDs in all follow-ups were found to be statistically non-significant; nevertheless, the observed SMDs in all included studies for the 3-, 6-, and 12-month follow-up were found to be negative (91.67%), which showed that the rise in a score of GMFM-66 was attributable to stem cells transplantation. The observed SMDs in all of the included studies, along with the 3-, 6-, 12-, and 24- month follow-up for GMFM-88, were found to be negative (100%), which suggested that the rise in the score of this outcome measure was attributable to stem cells transplantation. Although the SMDs in all of the follow-ups were statistically insignificant, the observed SMDs in all included studies were negative.
Despite this, the total effect size that was examined indicated, when comparing the administration of stem cells to the control group, that the control group had demonstrated a non- significant elevation in PEDI scores at the 5% level of significance in 12-month follow-up as (SMD: -0.59, 95% CI: -0.92 to -0.25, Pvalue: 0.001, 0.05, I2: 86.42%). The fact that the observed SMDs in all of the included studies and the 3-, 6-, and 12-month follow-ups were negative showed that the rise in PEDI scores resulted from stem cells being transplanted. This was shown to be the case in 85.71% of the cases. Except for the 3-month follow-up value (I2=0%), there was a high heterogeneity for most outcome measures for all follow-ups, the minimum value being I2=86.42% (the values of I2 for follow-ups of other parameters can be seen in Figure 1-3). This reflects the diverse patient and study characteristics and needs to be considered while discussing the results. A contributing factor may be that the type (mesenchymal stem cells, olfactory ensheathing cells, erythropoietin, mononuclear cells and neural progenitor cells) and source (adipose, fetal brain, umbilical cord, cord blood and Wharton's Jelly) of the transplanted cells differed in the included trials. While conducting this meta-analysis, it was noticed that the data for control was the same in the two studies [17,23]. However, the two articles were still considered for analysis as the cell types reported in both varied (UCT-MSC and UC MNC, respectively).
Conclusion
Conclusively, allogeneic stem cell administration for patients with cerebral palsy positively affects gross motor function compared to conventional therapies and rehabilitation.
Future Recommendations
Cerebral palsy is a complex ailment that requires further research using strategies focused on CP patients of different GMFCS levels, especially studies including placebo controls and other outcome measures to improve treatment options.
Authors' Contributions
SS searched extracted data, formulated the tables and wrote the first draft of the manuscript. DLG gave the basic concept and design of the study and proofread the manuscript. UEH assisted in the diagrams and proofreading of the manuscript. KA conducted the statistical analysis and assisted in the technical review of statistical analysis and interpretation. NK supervised the team, revised and approved the final version of the manuscript. AU helps in the collection of data and study material.
Availability of Data
All supporting documentation and data can be provided.
Compliance with Ethical Standards
This study did not include human participants, so no ethical authorization was required.
Consent for Publication
All the authors have no reservations about publishing this manuscript.
Conflict of Interest
The authors affirm that there are no conflicts of interest.
References
- Eggenberger S, Boucard C, Schoeberlein A, Guzman R, Limacher A, et al. (2019) Stem cell treatment and cerebral palsy: Systemic review and meta-analysis. World J Stem Cells 11: 891–903.
[Crossref], [Google Scholar], [Indexed]
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, et al. (2007) A report: The definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 49: 8–14.
[Google Scholar], [Indexed]
- Korzeniewski SJ, Slaughter J, Lenski M, Haak P, Paneth N, et al. (2018) The complex etiology of cerebral palsy. Nat Rev Neurol 14: 528–543.
[Crossref], [Google Scholar], [Indexed]
- McDonald CA, Fahey MC, Jenkin G, Miller SL (2018) Umbilical cord blood cells for the treatment of cerebral palsy; timing and treatment options. Pediatr Res 83: 333–344.
[Crossref], [Google Scholar], [Indexed]
- Sadowska M, Sarecka-Hujar B, Kopyta I (2020) Cerebral palsy: Current opinions on definition, epidemiology, risk factors, classification and treatment options. Neuropsychiatr Dis Treat 16: 1505–1518.
[Crossref], [Google Scholar], [Indexed]
- Alotaibi M, Long T, Kennedy E, Bavishi S (2014) The efficacy of GMFM-88 and GMFM-66 to detect changes in gross motor function in children with Cerebral Palsy (CP): A literature review. Disabil Rehabil 36: 617–627.
[Crossref], [Google Scholar], [Indexed]
- Vargus-Adams JN, Martin LK, Maignan SH, Klein AC, Salisbury S, et al. (2011) The GMFM, PEDI, and CP-QOL and perspectives on functioning from children with CP, parents, and medical professionals. J Pediatr Rehabil Med 4: 3–12.
[Crossref], [Google Scholar], [Indexed]
- Haley SM, Coster WI, Kao YC, Dumas HM, Fragala-Pinkham MA, et al. (2010) Lessons from use of the Pediatric Evaluation of Disability Inventory (PEDI): Where do we go from here? Pediatr Phys Ther 22: 69-75.
[Crossref], [Google Scholar], [Indexed]
- Mcintyre S, Goldsmith S, Webb A, Ehlinger V, Sandra JH, et al. (2022) The global prevalence of cerebral palsy: A systematic analysis. Dev Med Child Neurol 64: 1494–1506.
[Crossref], [Google Scholar], [Indexed]
- Bax M, Tydeman C, Flodmark O (2006) Clinical and MRI correlates of cerebral palsy: The european cerebral palsy study. JAMA 296:1602-1608
[Crossref], [Google Scholar], [Indexed]
- Zheng XR, Zhang SS, Yin F, Tang JL, Yang YJ, et al. (2012) Neuroprotection of VEGF-expression neural stem cells in neonatal cerebral palsy rats. Behav Brain Res 230: 108–115.
[Crossref], [Google Scholar], [Indexed]
- Lv ZY, Li Y, Liu J (2021) Progress in clinical trials of stem cell therapy for cerebral palsy. Neural Regen Res 16: 1377–1382.
[Crossref], [Google Scholar], [Indexed]
- Ben-David U, Benvenisty N (2011) The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer 11: 268–277.
[Crossref], [Google Scholar], [Indexed]
- Min K, Song J, Kang JY, Ko J, Ryu JS, et al. (2013) Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: A double-blind, randomized, placebo-controlled trial. Stem Cells 31: 581–591.
[Crossref], [Google Scholar], [Indexed]
- Chen L, Huang H, Xi H, Xie Z, Liu R, et al. (2010) Intracranial transplant of olfactory ensheathing cells in children and adolescents with cerebral palsy: A randomized controlled clinical trial. Cell Transplant 19: 185–191.
[Crossref], [Google Scholar], [Indexed]
- Xie B, Chen M, Hu R, Han W, Ding S, et al. (2020) Therapeutic evidence of human mesenchymal stem cell transplantation for Cerebral Palsy: A meta-analysis of randomized controlled trials. Stem Cells Int 2020: 5701920
[Crossref], [Google Scholar], [Indexed]
- Amanat M, Majmaa A, Zarrabi M, Nouri M, Akbari MG, et al. (2021) Clinical and imaging outcomes after intrathecal injection of umbilical cord tissue mesenchymal stem cells in cerebral palsy: A randomized, double-blind sham-controlled clinical trial. Stem Cell Res Ther 12: 1–15.
[Crossref], [Google Scholar], [Indexed]
- Hu K, Wang R, Xiao B, Gu Y, Liu F, et al. (2022) Effect of adipose-derived mesenchymal stem cell transplantation in five patients with cerebral palsy: A case report. Am J Transl Res 6: 33–40.
- Lv Z, Li Y, Wang Y, Li X, Han C, et al. (2022) Safety and efficacy outcomes after intranasal administration of neural stem cells in cerebral palsy: A randomized controlled trial.
[Crossref], [Google Scholar]
- Min K, Suh MR, Cho KH, Park W, Kang MS, et al. (2020) Potentiation of cord blood cell therapy with erythropoietin for children with CP: A 2 × 2 factorial randomized placebo-controlled trial. Stem Cell Res Ther 11: 1–12.
[Crossref], [Google Scholar], [Indexed]
- Sun JM, Case LE, Mikati MA, Jasien JM, McLaughlin C, et al. (2021) Sibling umbilical cord blood infusion is safe in young children with cerebral palsy. Stem Cells Transl Med 10: 1258–1265.
[Crossref], [Google Scholar], [Indexed]
- Sun JM, Case LE, McLaughlin C, Burgess A, Skergan N, et al. (2022) Motor function and safety after allogeneic cord blood and cord tissue-derived mesenchymal stromal cells in cerebral palsy: An open-label, randomized trial. Dev Med Child Neurol 64: 1477–1486.
[Crossref], [Google Scholar], [Indexed]
- Zarrabi M, Akbari MG, Amanat M, Majmaa A, Moaiedi AR, et al. (2022) The safety and efficacy of umbilical cord blood mononuclear cells in individuals with spastic cerebral palsy: A randomized, double-blind sham-controlled clinical trial. BMC Neurol 22: 1–13.
[Crossref], [Google Scholar], [Indexed]
- Luan Z, Liu W, Qu S, Du K, He S, et al. (2012) Effects of neural progenitor cell transplantation in children with severe cerebral palsy. Cell Transplant 21: S91–S98.
[Crossref], [Google Scholar], [Indexed]
- Kang M, Min K, Jang J, Kim SC, Kang MS, et al. (2015) Involvement of immune responses in the efficacy of cord blood cell therapy for cerebral palsy. Stem Cells Dev 24: 2259–2268.
[Crossref], [Google Scholar], [Indexed]
- Huang L, Zhang C, Gu J, Wu W, Shen Z, et al. (2018) A randomized, placebo-controlled trial of human umbilical cord blood mesenchymal stem cell infusion for children with cerebral palsy. Cell Transplant 27: 325–334.
[Crossref], [Google Scholar], [Indexed]
- Fu X, Hua R, Wang X, Wang P, Yi L, et al. (2019) Synergistic improvement in children with cerebral palsy who underwent double-course human Wharton's Jelly stem cell transplantation. Stem Cells Int 7481069.
[Crossref], [Google Scholar], [Indexed]
- Gu J, Huang L, Zhang C (2020) Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: A randomized, controlled trial. Stem Cell Res Ther 11: 43.
[Crossref], [Google Scholar], [Indexed]
- DerSimonian R, Laird N (2015) Meta-analysis in clinical trials revisited. Contemp Clin Trials 45: 139–145.
[Crossref], [Google Scholar], [Indexed]
- Şahin M, Aybek E (2019) Jamovi: An easy-to-use statistical software for the social scientists. Int J Assess Tool Educ 6: 670–692.
[Crossref], [Google Scholar]
- Boruczkowski D, Zdolińska-Malinowska I (2019) Wharton's Jelly mesenchymal stem cell administration improves the quality of life and self-sufficiency in children with cerebral palsy: Results from a retrospective study. Stem Cells Int 2019: 7402151.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences