Evaluating the Feasibility of Rolling out Universal Hearing Screening for Infants in India using Sohum, an Artificial Intelligence-driven Low Cost Innovative Diagnostic Solution
Arvind Vashishta Rinkoo, Anand Panjiyar, Dinesh Songara, Shivani Bhandari, Arnika Sharma, Meenakshi Pareek, Rajesh Ranjan Singh and Rakesh Kumar Srivastava
The Wadhwani Initiative for Sustainable Healthcare (WISH), New Delhi, India
- *Corresponding Author:
- Dr. Arvind Vashishta Rinkoo
Project Director, Innovations
The Wadhwani Initiative for Sustainable Healthcare (WISH)
New Delhi-110020, India
Tel: +919540318991
E-mail: arinkoo@wishfoundationindia.org
Received Date: October 05, 2019; Accepted Date: October 22, 2019; Published Date: October 29, 2019
Citation: Rinkoo AV, Panjiyar A, Songara D, Bhandari S, Sharma A, et al. (2019) Evaluating the Feasibility of Rolling out Universal Hearing Screening for Infants in India Using Sohum, An Artificial Intelligence-Driven Low Cost Innovative Diagnostic Solution. J Child Dev Disord. Vol.5 No.4:11
Abstract
This prospective study was undertaken with an objective to measure agreement between Sohum, an artificial intelligence-driven low cost innovative diagnostic solution, and the conventional Brainstem Evoked Response Audiometry (BERA) on various parameters of diagnostic importance and to investigate feasibility of Sohum as a universal hearing screening modality in India for neonates and infants. Our results statistically validate that the waveform generated by Sohum is clinically at par with that generated using BERA. Moreover, Sohum smartly obviates existing challenges to universal hearing screening implementation in India such as huge infrastructural requirement, limited capacity of frontline health workers, and high costs of contemporary hearing screening modalities such as conventional BERA. Additionally, as implementation of Sohum scales up across geographies and diverse demographic segments, its artificial intelligence-driven diagnostic algorithm would progressively become more robust and efficient.
Keywords
Hearing screening; Deafness; Sohum; India; Artificial intelligence
Introduction
The estimated incidence of permanent congenital or early-onset hearing impairment in developing countries-six cases per 1000 live births-is three times higher than in developed countries. Hearing defects in resource constraint settings lie on the high side, owing to a much greater incidence of low birth weight babies, consanguineous marriages, and morbidities such as jaundice, meningitis, etc. among newborns and infants in these settings. According to the World Health Organization, it is estimated that about 360 million individuals in the world have disabling hearing loss, of which 91% are adults and 9% are children. Hearing loss is the second most common cause of years lived with disability (YLD), accounting for 4.7% of the total YLD. The majority of people with disabling hearing loss live in low- and middle-income countries [1].
Unfortunately, hearing impairment is a serious but grossly neglected morbidity in India. In India, hearing impairment is second in prevalence among different types of disabilities. As per the 58th round of the National Sample Survey in 2002, 291 persons per 100,000 populations are suffering from severe to profound hearing loss across the country. In the same survey, about 32% of the people had profound (person could not hear at all or could hear only loud sounds) and 39% had severe hearing disability (person could hear only shouted words). Markedly, of these, a large percentage was children aged 0-14 years [2]. To put things in perspective, out of every 1000 children born in India, there are 5-6 children who cannot hear properly. As per census 2011, 23% of the disabled children (0-6 years) in the country are having disability in hearing.
It is well documented that first year of life is a crucial time for speech and language development in children. Hearing loss can have multiple deleterious effects on young children, which are not only related to attainment of speech and language but also to their social, emotional, and academic achievements. In fact, as per widely cited study of Olusanya, Neumann, and Saunders, interpersonal communication, psychological wellbeing, quality of life, and economic independence of individuals suffering with hearing disability are enormously affected [3]. Early and appropriate intervention for hearing loss in neonates can prevent critical developmental problems and would ensure optimal cognitive development in these children later in life [4-6].
Given these factors, universal newborn hearing screening has been adopted in many developed countries such as USA, UK, Canada, Australia, etc. [7,8]. Though the importance of early intervention in hearing loss among children is well documented in the literature, hearing screening in neonates remains unimplemented in India, owing to lack of infrastructural requirements such as noise-proof rooms, high cost, and requirement of highly skilled healthcare workers. At present, recommended technology for hearing screening of neonates in India is OAE (oto-acoustic emissions) as per the extant operational guidelines of the Ministry of Health & Family Welfare, Government of India for the National Programme for Prevention and Control of Deafness [9,10]. Notably, OAE requires a noise proof room for best results, thus considerably driving up the costs of setting up hearing screening programs. Another point of concern about the OAE technology is its lack of diagnostic accuracy as a screening modality. As per published literature, the sensitivity of OAE is 80% and specificity is 92.8%. This means that OAE screening would miss 20% hearing impaired children and falsely refer around 7%. Another study in 2014 found OAE referral rate at as high as 22% in a population with an actual incidence of 0.1-0.6% [11,12]. Thus, there is a growing body of evidence to suggest that OAE may not be viable as a universal hearing screening tool for neonates in developing countries such as India. The gold standard diagnostic modality for screening neonates and infants for deafness-Brainstem Evoked Response Audiometry (BERA) is out of question in context of a developing country such as India because of huge infrastructural costs involved and limited capacity of primary healthcare workers. Sohum’s indigenously developed BERA-based hearing screening device was innovated keeping in mind infrastructural challenges, limited capacity of frontline healthcare workers, and low accuracy and high costs of current hearing screening modalities. The portable and compact, battery-driven device does away with the need of a noise-proof room and thus, reduces infrastructural costs and brings the test to the bedside of the child. This study was undertaken with an objective to measure agreement between Sohum and the conventional diagnostic BERA on various parameters of diagnostic importance and to investigate feasibility of Sohum as a universal hearing screening tool for neonates and infants.
Research Methodology
Study setting
This prospective study was conducted at a tertiary care hospital in India for duration of two months during the period March 2019 to April 2019. The primary objective of the study was to measure agreement of an audiologist on various parameters of diagnostic importance in the waveforms generated by Sohum and the conventional diagnostic BERA, and to investigate feasibility of Sohum as a universal hearing screening tool for infants in terms of cost, convenience, and quality. Assuming the sample size to give a Cohen’s kappa value of 0.9 with the maximum acceptable 95% confidence interval width of 0.3 and the true proportion of positives to be 40%, the required sample size came out to be 34 waveforms in order to statistically validate the agreement between Sohum and the conventional diagnostic BERA [13]. Additionally, during the study period, around 300 ears of neonates and infants were screened for deafness using Sohum to evaluate feasibility of Sohum as a universal hearing screening tool.
Each child, aged 0-12 months, who were advised BERA by the treating doctor based on comprehensive history taking and thorough clinical examination underwent the two tests-diagnostic BERA and Sohum-on both the ears. An experienced audiologist, completely blinded to the type of diagnostic modality used and the identity of the patient, reviewed all the waveforms generated and reported pre-defined outcomes [presence of peak I in the waveform (yes/no), presence of peak III in the waveform (yes/ no), and presence of peak V in the waveform (Yes/No)] for each of the waveforms generated using BERA and Sohum.
Simultaneously, around 300 neonates and infants who visited the tertiary care hospital and who were advised hearing screening by the treating doctor based on comprehensive history taking and thorough clinical examination underwent Sohum diagnostic test to gauge feasibility of Sohum as a universal hearing screening tool in terms of cost, convenience, and quality.
The equipment
Sohum hearing screening device (Figures 1 and 2) was developed to address concerns of low diagnostic accuracy, and of requirement of noise proof rooms and of skilled health workers. It is portable and compact, easy to use device. The technology uses selective artefact rejection that eliminates use of sedatives and enables usage in noisy environments. Moreover, the underlying technology is based on a reusable, easy to clean electrode system. Its artificial intelligence-driven unique algorithm provides high sensitivity and specificity, well-proven in clinical trials. Sohum hearing screening test has an optimized design that reduces test duration by minimizing time for preparation and analysis, and makes the test ideal for hearing screening in children. Besides, Sohum test screening procedure is extremely simple, enabling task shifting from skilled to unskilled healthcare workers. Sohum screening device was launched in July 2017. At present, it is already in use in 16 clinical centres across seven cities in India with more than 5000 screenings having been successfully conducted. The Government of Tripura and the Government of Andhra Pradesh have partnered with the Sohum technologies. Multiple pilot/validation studies have been conducted in different settings across the country to corroborate Sohum’s feasibility, efficacy, and safety as a universal hearing screening modality for children.
In BERA, for electrode application, the skin of the baby is cleaned by a gentle abrasive skin preparation gel. The positive electrode is placed on the vertex at the midline, the negative electrode on the mastoid, and the ground electrode on the opposite ear. The impedance is kept to a minimum (<5 kΩ). The signal is delivered through insert earphones. The stimulus used is click signal, presented at various intensities to obtain the air conduction threshold of the ear. The threshold of hearing is taken as the lowest intensity at which a replicable wave V is obtained. The results are recorded and used for analysis.
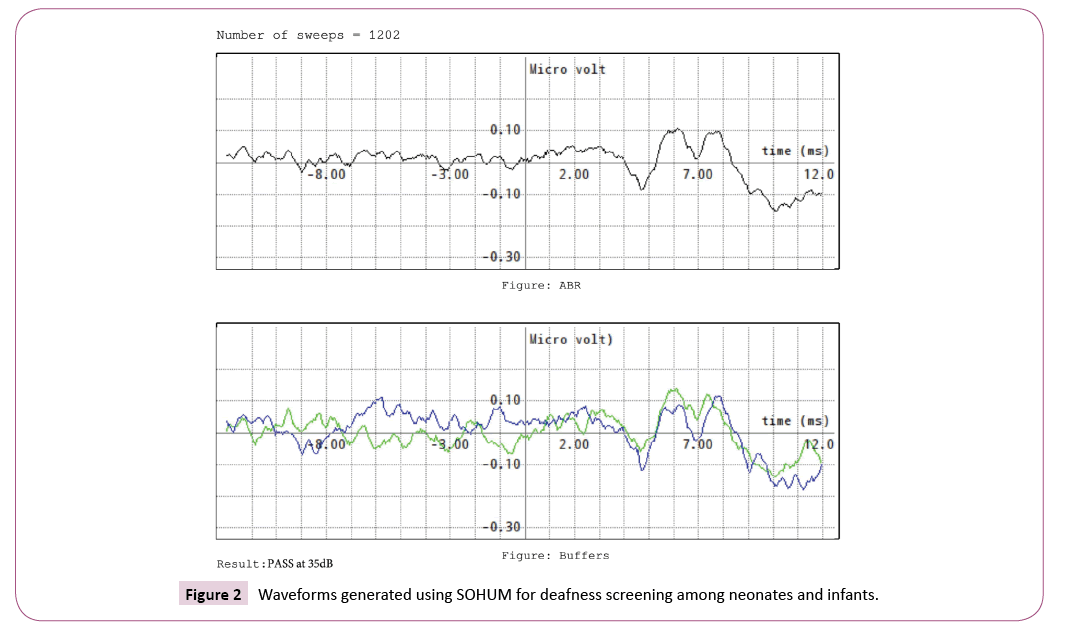
In Sohum, the identified children would undergo the screening test with click stimuli presented at 35/50/70/90 decibels. The criteria used for passing a neonate are reproducibility of waveform, presence of peak III at 35 decibel, and classifiers to detect patterns. The refer/pass results and the waveforms are recorded.
Statistical Analysis
Cohen's kappa coefficient (κ) was calculated to measure the degree of agreement on various categorical variables. IBM SPSS Statistics version 24 was used for basic statistics, whereas Statistical MedCalc Version 18.10 was used for advanced statistics.
Ethical Considerations
The institutional ethical committee of LEHS|WISH approved this study (WISH/IEC/2018/1-6). Written informed consent was taken from the guardians of all the subjects before enrolling them in this study. Those who did not give written informed consent were excluded from this study.
Results
During the study period, a total of 332 ears of neonates and infants were screened for deafness using Sohum. These infants visited the tertiary care hospital during the study period and were advised hearing screening by the treating doctor based on comprehensive history taking and thorough clinical examination. Details are shared in Table 1.
Table 1 Number of ears of neonates/infants screened for deafness using SOHUM.
| Age groups | Number of ears screened using SOHUM |
|---|---|
| 0-1 month (Neonates) | 204 (61.44%) |
| 1-6 months | 114 (34.34%) |
| 6-12 months | 14 (4.22%) |
| Total | 332 |
Table 2 gives details about the neonates/infants who were screened for deafness using Sohum during the study period. The details include percentage of boys/girls screened for deafness using Sohum, mean age of neonates/infants screened (in months), mean weight of neonates/infants screened (in grams), and mean gestational age of mother at delivery (in months).
Table 2 Details about the neonates/infants who were screened for deafness using SOHUM during the study period.
| Parameters | Value |
|---|---|
| Number of ears screened using SOHUM | 332 |
| Boys/Girls (in %) | 65.70%/34.30% |
| Mean age in months(95% CI) | 1.44 ± 1.82 (1.16-1.72) |
| Mean weight in grams (95% CI) | 2407.83 ± 1092.98 (2240.34-2575.33) |
| Mean gestational age of mother at delivery in months (95% CI) | 8.44 ± 0.84 (8.31-8.57) |
CI: Confidence Interval; All continuous variables are expressed as mean ± standard deviation
Table 3 details the results of Sohum tests of neonates/infants who were screened for deafness during the study period. The click stimuli for Sohum test were presented at 35/50/70/90 decibels. Among the total study sample of 332 ears, 2 ears were found to be profoundly deaf (refer at 90 decibel), 17 ears were moderately deaf (refer at 50 and/or 70 decibels), and 19 ears were mildly deaf (refer at 35 decibel). However, during the study period, as many as 80.91%, 72.40%, 43.97%, and 55.73% of ears were classified under the “redo/verify” group by the Sohum algorithm at 90 decibel, 70 decibel, 50 decibel, and 35 decibel, respectively.
Table 3 Results of SOHUM tests of neonates/infants who were screened for deafness during the study period.
| Results | Stimuli presented at 35/50/70/90 decibels | |||||||
|---|---|---|---|---|---|---|---|---|
| 35 decibels | 50 decibels | 70 decibels | 90 decibels | |||||
| Number | Percentage | Number | Percentage | Number | Percentage | Number | Percentage | |
| Pass/Normal | 120 | 38.22% | 134 | 52.14% | 62 | 24.80% | 44 | 18.26% |
| Redo/Verify | 175 | 55.73% | 113 | 43.97% | 181 | 72.40% | 195 | 80.91% |
| Refer/Abnormal | 19 | 6.05% | 10 | 3.89% | 7 | 2.80% | 2 | 0.83% |
| Total Ears | 314 | 257 | 250 | 241 | ||||
Simultaneously, during the study period, forty waveforms of Sohum and diagnostic BERA were compared by an experienced audiologist. The audiologist, completely blinded to the type of diagnostic modality used and the identity of the patient, reviewed all the waveforms generated and reported pre-defined outcomes [presence of peak I in the waveform (yes/no), presence of peak III in the waveform (yes/no), and presence of peak V in the waveform (Yes/No)] for each of the waveform. Table 4 gives the degree of agreement of the audiologist on various categorical variables in the waveforms generated using Sohum and the diagnostic BERA in terms of Cohen's kappa coefficient (κ).
Table 4 Degree of agreement of the audiologist on various categorical variables in the waveforms generated using SOHUM and the diagnostic BERA.
| S. No. | Parameters (Categorical variables) | Cohen's Kappa Coefficient (κ*) |
|---|---|---|
| 1. | Absence of peak I | Κ=1.0000 |
| 2. | Absence of peak III | Κ=0.8666 (95% CI: 0.6869 to 1.0000) |
| 3. | Absence of peak V | Κ=0.7109 (95% CI: 0.6869 to 0.9434) |
*κ takes into account the possibility of the agreement occurring by chance; agreement is: poor if k < 0.001, slight if 0.00 ≤ k ≤ 0.20, fair if 0.21 ≤ k ≤ 0.40, moderate if 0.41 ≤ k ≤ 0.60, substantial if 0.61 ≤ k ≤ 0.80, and almost perfect if k > 0.80 [13]; CI: Confidence Interval
Discussion
Existing literature corroborates that effective screening of the newborns and infants is the most cost-effective way to reduce the burden of hearing loss. “Catch them young” should be the central theme of any program for the control of deafness in developing countries such as India [6,14-16]. However, extant guidelines in India recommend that screening of neonates and infants be done using OAE, which lacks both sensitivity and specificity as a modality for screening deafness [9,11,12]. This guideline is corollary of the fact that using the gold standard diagnostic BERA for universal screening of newborns and infants in a developing country such as India is neither plausible nor cost-effective.
Given this context, the findings of this study, though preliminary, could serve as a foundation to inform public health policymakers to consider Sohum as a means to roll out universal hearing screening for neonates and infants in India. As per our study, there is “substantial to almost perfect” agreement [in terms of Cohen's kappa coefficient (κ)] of the audiologist on various categorical variables [presence of peak I (Yes/No), presence of peak III (yes/no), and presence of peak V (yes/no)] in the waveforms generated using Sohum and the diagnostic BERA. Thus, the present study statistically validates that the waveform generated by Sohum is clinically at par with the waveform generated using diagnostic BERA. At the same time, Sohum’s indigenously developed BERAbased innovative hearing screening device potentially obviates existing challenges such as huge infrastructural requirement, limited capacity of frontline health workers, and high costs of existing hearing screening modalities such as conventional BERA.
However, in our study, as many as 80.91%, 72.40%, 43.97%, and 55.73% of ears of neonates and infants were classified under the “redo/verify” group by Sohum automated system at click stimuli of 90 decibel, 70 decibel, 50 decibel, and 35 decibel, respectively. At a click stimuli of 90 decibel, only 18.26% ears were classified under “pass” [normal] and 0.83% ears were classified under “refer” [abnormal and hence needing a referral to an otolaryngologist]. The remaining 80.91% of ears were classified under the “redo/verify” group by the Sohum algorithm.
Conclusion
The current artificial intelligence-driven diagnostic algorithm of Sohum needs to be more robust. Interestingly, this issue would be largely addressed if the innovator can reclassify the “verify” cases as “pass subject to verification”. Additionally, as more quality data pours in, robustness of the artificial intelligencedriven Sohum diagnostic algorithm would progressively improve over time. In very near future, Sohum-driven universal hearing screening programme for neonates and infants has the potential to revolutionize prevention and control of deafness in India.
Disclaimer
The opinions or views expressed in this article are solely those of the authors and do not necessarily express the views or opinions of the organization to which the authors are affiliated.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgement
The Sohum project is supported by funds from Biotechnology Industry Research Assistance Council (BIRAC), Department of Biotechnology (DBT), Government of India (DBT sanction order number BFD/AO/AO5.05Q/155/17-18).
References
- World Health Organization (2015) Deafness and Hearing loss-Fact Sheet 2015.
- National Sample Survey Organization (2003) Disabled Persons in India. New Delhi: Ministry of Statistics and Programme Implementation. Government of India; December 2003. Report No. 485.
- Olusanya BO, Neumann KJ, Saunders J (2014) The global burden of disabling hearing impairment call to action: A call to action. Bulletin of the World Health Organization 2014, 92: 367-373.
- Abramovich SJ, Hyde ML, Riko K, Alberti PW (1987) Early detection of hearing loss in high risk children using brain stem electrical response audiometry. J Laryngol Otol 101: 120-126.
- Augustine AM, Jana AK, Kuruvilla KA, Danda S, Lepcha A, et al. (2014) Neonatal hearing screening: Experience from a tertiary care hospital in Southern India 2014. Indian Pediatr 51: 179-183.
- Garg S, Singh R, Khurana D (2015) Infant hearing screening in India: Current status and way forward. Int J Prev Med 6: 113.
- White KR (1997) Universal new-born hearing screening issues and evidence. CDC Workshop on Early Detection and Intervention of Hearing Impairments in new-borns. Atlanta, Georgia, Pp: 105.
- World Health Organization (2010) Newborn and infant hearing screening: Current issues and guiding principles for action. World Health Organization, Switzerland, Geneva.
- Directorate General of Health Services, Government of India (2013) Guidelines of the National Programme for Prevention and Control of Deafness (NPPCD). New Delhi, India: Government of India p: 32.
- Kishore J (2015) National health programs of India. Delhi: Century publications (11th edn).
- Kumar S, Mohapatra B (2011) Status of newborn hearing screening program in India. Int J Pediatr Otorhinolaryngol 75: 20-26.
- Nagapoornima P, Ramesh A, Srilakshmi SL, Rao S, Patricia PL, Gore M, et al. (2007) Universal hearing screening. Indian J Pediatr 74: 545-549.
- Watson PF, Petrie A (2010) Method agreement analysis: a review of correct methodology. Theriogenology 73: 1167-1179.
- Vignesh SS, Jaya V, Sasireka BI, Sarathy K, Vanthana M (2015) Prevalence and referral rates in neonatal hearing screening program using two step hearing screening protocol in Chennai-A prospective study. Int J Pediatr Otorhinolaryngol 79: 1745-1747.
- Sokol J, Hyde M (2002) Hearing Screening. Pediatr in Rev 23: 155-162.
- Sood M, Kaushal RK (2009) Importance of new-born hearing screening. Indian J Otolaryngol Head Neck Surg 61: 157-159.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences