Arteriovenous Malformation in a Youth with Atypical Autism Symptoms
Veena Sison,Tracy Stackhouse,Robert Breeze,Terry Hall ,Pamela McKenzie and Nicole Tartaglia.
DOI10.4172/2472-1786.100042
Veena Sison1-3, Tracy Stackhouse4, Robert Breeze5, Terry Hall2,6, Pamela McKenzie1,2 and Nicole Tartaglia1*,2
1Developmental Pediatrics, Children’s Hospital Colorado, Aurora, CO, USA
2Department of Pediatrics, University of Colorado School of Medicine, Aurora, CO, USA
3Southern California Kaiser Permanente Medical Group, Woodland Hills, CA, USA
4Developmental FX, Denver, CO, USA
5Department of Neurosurgery, University of Colorado School of Medicine, Aurora, CO, USA
6JFK Partners, University of Colorado School of Medicine, Aurora, CO, USA
- *Corresponding Author:
- Nicole Tartaglia
Associate Professor of Pediatrics
University of Colorado
School of Medicine 1312
3 East 16th Ave, B140, Aurora, USA
Tel: 720-777-8087
E-mail: Nicole.tartaglia@childrenscolorado.org
Received Date: February 14, 2017; Accepted Date: February 15, 2017; Published Date: February 28, 2017
Citation: Sison V, Stackhouse T, Breeze R, et al. Arteriovenous Malformation in a Youth with Atypical Autism Symptoms. J Child Dev Disord. 2017, 3:4. doi:10.4172/2472-1786.100042
Abstract
Cerebral arteriovenous malformations (AVMs) present a challenge to diagnose in children with developmental disability, because of the overlap in behavioural symptoms and neurologic manifestations. They have been very rarely reported in conjunction with autism spectrum disorder. This case involves a 13 year old male with a history of autism spectrum disorder and significant behavioural issues diagnosed with a thalamic AVM following lateralizing neurologic symptoms. Despite radiosurgical treatment, hemorrhage followed consequently causing extensive neurologic injury and death. This case emphasizes the need for close follow up and coordination within a medical home for children with developmental disabilities. A multidisciplinary team approach is ideal to allow detection of subtle neurologic changes over time that may be masked as behavioural difficulties.
Keywords
Malformation; Autism symptoms; Arteriovenous
Introduction
Pediatric Arteriovenous Malformations (AVMs) are rare vascular abnormalities consisting of fistulous connections of arteries and veins vessels without a normal intervening capillary bed. Shunting of arterial blood and pressure on the venous vessels build, which may lead to increased size of the AVM, and possible rupture with either small or large intracranial bleeds. Subsequent neurologic sequelae vary with the location of the AVM and the severity of the bleeding following rupture. Sometimes, large AVMs interfere with circulation of cerebrospinal fluid, resulting in hydrocephalus and increased intracranial pressure [1]. The prevalence rate in children and adults is estimated from 1 to 10.3 in 100,000 [2], and most are asymptomatic or identified incidentally with neuroimaging [3]. Compared with adults, children with AVMs are more likely to present with hemorrhage and mortality rates from bleeding as high as 25% have been reported [4]. If an AVM is identified prior to a major hemorrhage, medical emergencies can be prevented and interventions are more likely to be effective. See Table 1 for possible signs of an AVM prior to a hemorrhage.
| Signs and Symptoms of Cerebral AVM |
| Mental status/Cognitive changes: Mental confusion Difficulty with speech production (dysarthria) or language/communication (aphasia) Memory deficits Hallucinations |
| Sensory changes: Unusual sensations Buzzing in the ears (pulsatile tinnitus) Numbness or tingling (face or any part of body) Spontaneous idiopathic pain (paresthesia or dysesthesia) |
| Motor changes: Muscle weakness or paralysis (face or any part of body) Loss of balance/motor coordination (ataxia) Unusual gait Poor motor planning (apraxia) |
| Other: Headache Seizures (all types/varying severity) |
| Depending on which part of the brain is most pressured: Vision abnormalities (blurred, double, or worsening acuity) Difficulty with visual tracking (coordinating eye movements) Dizziness Numbness |
Table 1 Signs and symptoms of cerebral AVM.
There is little in the literature that connects AVMs to Autism Spectrum Disorder (ASD). They can be challenging to detect in otherwise healthy children, hence, are even more difficult to identify in children with developmental impairments. First, some of the indicators in AVMs overlap with common features seen in children with ASD, such as language/communication difficulties, motor coordination deficits, unusual gait, sensory differences, and emotional symptoms that can fluctuate over the lifetime. Second, individuals with communication difficulties may not have the ability to communicate specifics of pain or discomfort or to explain more subtle neurologic symptoms that they are experiencing. Third, some children with ASD have difficulty tolerating medical appointments and procedures, leading to limited physical examinations and heightened thresholds of ordering medical tests due to distress caused to the patient and their parents.
In this report we present a case of a youth with developmental disability and ASD who presented with an AVM.
Case Report
Early developmental history
The patient was born to 29 year old professional parents. He was his mother’s second pregnancy and first live birth, with one previous miscarriage at 10 weeks gestation. The pregnancy was naturally conceived and there were no complications. Prenatal ultrasounds showed no abnormalities. He was delivered after 18 h of labor via Caesarian section for failure to progress. Birth weight was 7lb.12oz. Apgar scores were 8 and 9 at 1 and 5 minutes respectively. There were no complications or concerns in the neonatal period.
For the first 14 months of life, medical and developmental records report that he met expected developmental milestones. He first sat unsupported at 5 months, and first crawled at 9 months. His first single words occurred at 8 months, and by 14 months had a vocabulary of at least 10 different words, along with several communicative gestures, including pointing. At the time of his 14 month well-child visit with his paediatrician, there were no concerns related to social development or play skills. He was beginning to pull-to-stand, but was not yet walking independently. Height and weight and head circumference tracked around the 50th percentile from birth. He was a healthy infant, with the exception of multiple ear infections starting at 3 months of age treated with multiple courses of antibiotics. He did not require any hospitalizations or surgeries.
His parents relocated to another state at 15 months of age. During the 4 month period between 15-19 months of age, his parents noted a decline in his communication skills, social responsiveness, play behaviours, and participation in daily routines. Previously acquired expressive language and communication skills decreased or were lost completely, including gesturing and pointing. He began to avoid eye contact, especially with strangers. He did not feel as connected socially, and was increasingly wary of new people and hesitant about new experiences. He no longer tolerated being fed by another person and began to self-feed with his fingers, resisting attempts to increase his intake or vary his diet. He stopped stacking blocks and using toys in a functional manner; instead he began scattering toys haphazardly and showed less curiosity towards new toys. His gross motor skills progressed, however, and he began walking independently at 17 months of age. At 17 months of age, he experienced an acute febrile illness associated with 3 days of high fever and dehydration, from which he recovered with outpatient treatment. Developmental changes during this time were thus initially suspected to be related to the adjustments in his living situation and daily routines, as well as recovery from illness.
His development continued to plateau until 29 months, at which time formal developmental evaluation was completed. Hearing and vision screening were normal. Standardized developmental testing estimated his receptive language skills to be at the 10 month age equivalent, and expressive skills at approximately the 14 month age equivalent. Gross and fine motor skills ranged from the 15-18 month age equivalent, with impaired motor planning. It was noted that his impaired dexterity appeared to frustrate him and he often refused to participate in motor activities, both within testing situations and in daily life. His behavior during the evaluation was described as “mild-mannered, not very exploratory in play, affectionate with his parents, but less socially available with unfamiliar people.” He was reported to share with others, engage in parallel play with other children for brief periods, and respond to his name consistently. The occupational therapist noted that he showed unusual responses to sensation, sought excessive proprioceptive input, and had difficulties modulating his arousal level. Occupational and speech therapy were initiated at 30 months of age.
At 30 months of age he was evaluated at a University-based Children’s Hospital clinic by a Developmental-Behavioural Pediatrician and Child Psychologist. Developmental testing using the Bayley Scales of Infant Development–2nd Edition showed a Mental Development Index of less than 50, at the 14-16 month age equivalent. On the Childhood Autism Rating Scale, his score was 36.5 (cutoff for autism is 30). Behavioural observations noted in this evaluation included: Inconsistent social responsiveness, unusual responses to sensation, limited communication ability both non-verbally and verbally, and a strong preference for familiarity and routine. He exhibited self-stimulatory motor behaviours (spinning himself in circles) and visual behaviours (close visual inspection of objects), and toe-walking. Medically, the pediatrician noted episodes of staring, poor ocular control, and eye fluttering. Physical examination showed height at the 10th percentile, weight at the 50th percentile, and head circumference at the 50th percentile. There were no dysmorphic features or neurocutaneous findings noted. Neurologic examination reported intact and symmetric cranial nerves, normal muscle mass and strength, global hypotonia, poor balance, and impaired motor planning. Gait was wide-based and immature. The remainder of his physical examination was unremarkable. Impressions included severe sensory motor integration dysfunction and autism, with further recommendations for EEG/MRI, Occupational therapy, and medical evaluation.
Medical work-up between 30-33 months of age included a normal EEG, normal brain MRI, and normal Brainstem Auditory Evoked Potential results. Chromosome analysis showed a normal 46, XY male karyotype and Fragile X DNA testing was negative. Testing for metabolic disorders was also performed (including plasma amino acids, serum and urine organic acids), and these showed elevated serum lactic acid and possible increases in fumaric, malic, and 2-keto-glutaric acid. He was evaluated by the Inherited Metabolic Disease Clinic, where urine mucopolysaccharides and a repeat urine organic acid were recommended and showed normal results.
After these evaluations, he began an intensive home-based therapy program that included 10-20 hrs per week of Occupational therapy, Speech Therapy, and Behavioural/ Educational programming, in addition to clinic-based OT. With the onset of intervention, his expressive language slowly returned and communication improved. By 33 months of age, he was using 5 words on a regular basis; at 50 months, he began to use phrases consisting of 2-4 words. He began to receive public school special education services at age 3.
His family also enrolled him in a longitudinal study of the development of autism, funded through the NICHD Collaborative Programs of Excellence in Autism Network. Through this study, he completed evaluations at 35 and 50 months of age which included standardized developmental testing, the Autism Diagnostic Observation Schedule (ADOS), standardized adaptive behavior interviews, and the Autism Diagnostic Interview–Revised (ADI-R). He showed some improvement in the areas of visual reception, and both fine and gross motor skills over time on developmental testing, but showed a plateauing of both receptive and expressive communication skills. On adaptive testing, communication and socialization skills improved over time, but Daily Living Skills had minimal improvement. Across time points, he consistently fulfilled criteria for a diagnosis of autism based on the ADOS and ADI-R.
From age 3 to 11, he received intensive ASD programming and treatment, with both school-based and private therapies which included direct applied behavioural intervention techniques for ASD, speech therapy with augmentative communication strategies, and occupational therapy. He attended a private school specialized in education of students with ASD from age 8-11 years, at which time he transitioned back to the public school setting. He continued to undergo periodic developmental evaluations, both through private therapists and through the longitudinal autism study. He consistently showed delays across domains, but showed slow progress toward therapy goals and acquired new skills. There were periods over the years where he seemed to plateau in skills or have mild regressions, especially in his speech/communication abilities and general interest in social interactions. However, these periods would resolve after a few months with a more rapid acquisition of previously attained skills and improved social relatedness. He had strengths in visual spatial abilities and computer-based tasks, and he had a nice appreciation of music. He participated in many community-based activities for children with disabilities including soccer and swimming, and he enjoyed skiing and travelling with his family. He also received some alternative and complementary therapies during this period, including auditory integration training, chelation therapy, secretin treatments, music therapy, and treatment with various herbal formulations and homeopathics supplements.
At age 11, medication consultation was sought due to increasing behavioural difficulties which had been noted since the onset of puberty, including low frustration tolerance, increased separation anxiety, increased resistance to new activities or therapies, and an increase in self-injurious and aggressive behaviours (banging his head, pinching, hitting). At that time, he communicated using mostly single words or short phrases, with frequent stereotyped phrases or repetitive vocalizations. His receptive language abilities were notably stronger and he was still able to follow two step commands. He had strong interests in Thomas the Train and Sesame Street, with a stuffed Big Bird toy always carried with him.
He had significant fears of vacuum cleaners and garage doors, although over the past year had seemed more upset by loud noises such as loud vehicles, his baby sibling crying, or barking dogs. His mother described periods of sudden loud vocalizations and/or crying episodes, and periods where he would become agitated and hide his head under a pillow. He had previously been more flexible with new situations such as family travel or outings to restaurants; however these were becoming more difficult as well. At this time, he preferred sleeping in dark rooms during the day, and had decreased energy for physical activities. He also started having intermittent choking on soft foods. Examination showed height at the 50th percentile, weight at the 25th percentile, and head circumference at the 70th percentile. He had normal vital signs. He had tactile defensiveness with the examination, but he was able to tolerate it with behavioural and parental support. Additional behavioural observations included decreased eye contact, echolalia, periods of stereotypic motor movements of hands and arms while appearing to recite a script, and close visual inspection of objects. Neurologic examination was notable for mild facial asymmetry and global hypotonia. He had otherwise normal and symmetric cranial nerves, strength, muscle bulk, reflexes, and sensation. There were no other signs or symptoms of dental problems, otitis, gastroesophageal reflux, constipation, allergies, or other medical problems which can present as behavioural exacerbations in children with ASD. Treatment with a low dose of citalopram led to improvements in some of his symptoms, including improved self-regulation and decreased hitting/pinching when upset, decreased overall anxiety, and improved socialization. Updated genetic testing including chromosomal microarray and MECP2 gene sequencing showed normal results.
At age 12 years and 10 months, he was seen for evaluation due to concern for left leg weakness and gait asymmetry that had become apparent over the past few months. He had begun to drag his left foot when walking, which caused him to trip every few yards. His ski instructor had also noted that his left leg seemed weaker and kept turning out while skiing, and his endurance decreased from full day ski sessions to a few hours and eventually was not able to participate. He was also having increasing behavioural dysregulation, with outbursts and anxiety that were becoming increasingly difficult to manage during public family outings. Neurologic examination was remarkable for increased deep tendon reflexes in his left arm and leg, with persistent left ankle clonus and an up going left toe. Gait was notable for toe-walking and mild dragging of his left leg, with intermittent stumbling. Leg lengths were symmetric.
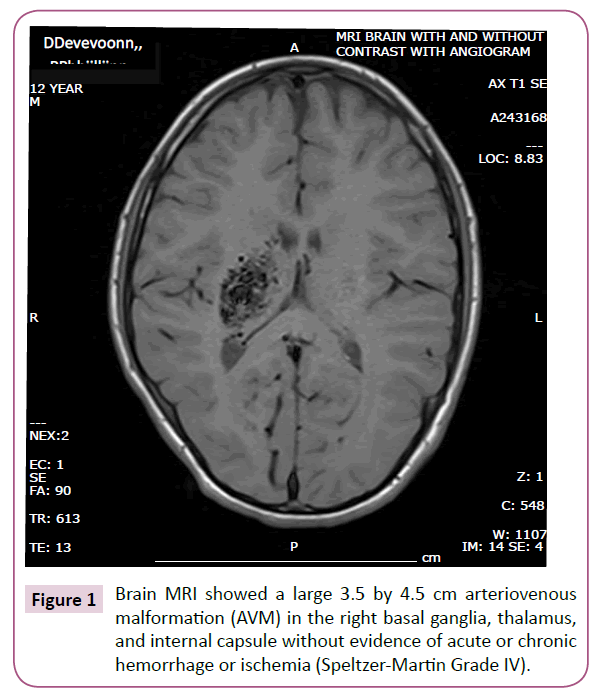
Brain MRI and subsequent cerebral angiogram showed a large 3.5 by 4.5 cm arteriovenous malformation (AVM) in the right basal ganglia, thalamus, and internal capsule without evidence of acute or chronic hemorrhage or ischemia (Speltzer-Martin Grade IV) which is shown in Figure 1. Findings were reviewed with several neurosurgeons and interventional radiologists. Treatment was favored because of progressive symptoms, however due to the deep and delicate location of the AVM, treatment involving surgery or embolization was considered very high risk.
At 13 years 4 months, he began a planned regimen of three gamma knife radiosurgery treatments to be spaced 6 months apart in attempt to decrease the AVM size. Between the first two treatments, his family reported improvements in his energy level and social relatedness. His left sided weakness progressed slightly. At 13 years 11 months, he developed acute onset of left-sided paralysis and progressively decreasing level of consciousness. Emergent head CT scan showed a large right thalamic hemorrhage at the site of the AVM, with intraventricular hemorrhage, enlarged ventricles, cerebral edema, and midline shift. His care providers and family members discussed his prognosis, and it was determined that aggressive life-saving measures may be futile, and he would have significant long-term neurologic injury and a poor quality of life. His family ultimately decided to treat with comfort care measures, and he passed away with his family at his bedside.
Discussion
This case emphasizes the complexities and importance of recognizing neurological symptoms in children and adolescents with autism spectrum disorders and limited language abilities. The patient demonstrated many neurologic symptoms likely caused by the AVM that were attributed to various behavioural aspects of ASD. Increased self-injurious behavior and behaviours such as hiding his head under a pillow, banging his head, or preferring dark rooms were likely due to increased auditory sensitivities, visual symptoms, and/or headache pain related to the AVM. Increased behavioural agitation/irritability and resistance to social outings with family were partially attributed to increased behavioural difficulties or anxiety that commonly occur with onset of adolescence in ASD, while there was likely a contribution of pain or other neuropsychologic sequelae of the AVM. Given that behaviours are grounded in communication, if a child cannot effectively communicate his needs, it becomes the responsibility of service providers to delve into understanding the underlying source of what these behaviours may be communicating. For many providers, negative behaviours such as self-injury or aggression are not always interpreted as having a physiologic basis.
Indicators of increased cranial nerve involvement included the progression of facial asymmetry which was not notable to a new behavioural care team due to progression over many years, as well as new difficulties with choking. It was not until clear neurologic symptoms such as asymmetric lower extremity weakness and gait abnormalities developed that further evaluation led to the identification of the AVM. This case demonstrates that a medical etiology should be carefully considered when individuals with ASD present with exacerbation of behavioural symptoms or with new behaviours inconsistent with their prior history rather than attributing these changes to the atypical behaviours common in ASD. It also highlights the need for multidisciplinary teams to be vigilant in observation and documentation of progress and changes in physical and developmental presentation. Any provider on a team should recommend medical evaluation if a noted behavioural change occurs that may have a physiologic basis [5].
It is notable that he had an MRI following his initial ASD diagnosis at 30 months of age which was read as normal. At the time of his AVM diagnosis, films from this original MRI were no longer available for review. While AVMs are vascular malformations assumed to be present at birth, they can increase in size over time and thus it is possible it was not yet visible or very small at the time of the original MRI. There have been case reports describing de novo cerebral AVMs in children who were symptomatic prior to their discovery via subsequent brain MRI [6,7]
The growth of his AVM over time, however, brings up speculation of the contribution of the AVM to earlier neurodevelopment changes. The location within the basal ganglia, thalamus and internal capsule which are brain areas containing pathways and interconnections of networks from multiple neural systems may have contributed to neurodevelopmental and sensory differences. Other earlier symptoms that are somewhat atypical of ASD such as fluctuations and regressions in language development and social interactions in the school age years may have also been related to the AVM. It is possible that hallucinations or other odd sensations triggered by the AVM contributed to the new-onset sudden behavioural outbursts and loud vocalizations, or new motor stereotypic movements.
There is limited literature reporting the co-occurrence of AVMs in children with ASD. Reported cases include individuals with PTEN mutations, which are associated with macrocephaly and other features of PTEN-associated disorders such as Cowden syndrome (which includes other features such as benign skin hamartomas, penile freckling in males, intestinal polyps and increased risk for specific cancers) [8]. More recently, PTEN mutations have been identified in children presenting with both autism spectrum disorders and macrocephaly [9].
This patient was not tested for a PTEN mutation; however it was unlikely due to a normal head circumference and lack of other physical findings or family history suggestive of PTEN mutations. In our literature search, there is only 1 other reported case of a child with ASD and cerebral AVM [7].
This patient received primary care at a well-respected pediatric practice; however medical evaluations were difficult for him due to sensory sensitivities and anxiety. Routine well child care appointments were more limited than in typically developing children, and did not allow for exploration of more complex topics such as changes in behavior. Similarly, he was evaluated by different providers when seen for acute illnesses, preventing identification of notable changes in appearance or function over time. Upon evaluation by a new developmental specialist in early adolescence, distinguishing baseline behavioural symptoms commonly associated with ASD versus progressive neurologic symptoms was challenging in a complex patient with a long history. For example, facial asymmetry progressed slowly over the years, thus to a new provider may not be interpreted as a neurologic sign since structural and functional facial asymmetry is not uncommon in neurodevelopmental disorders, and it was reported as being present for many years. Similarly, escalation of behavioural difficulties and new anxieties are common with the onset of adolescence in ASD.
Current treatment recommendations for children with ASD include treatment and yearly follow-up within a medical home as well as follow-up related more specifically to ASD symptoms and behaviours by a specialist in ASD such as developmental-behavioural pediatrics, psychiatry, or neurology [10,11]. This allows for less fragmented care so that changes over the years can be appreciated. Further, medical offices providing primary and specialty care to children with ASD and developmental disabilities are encouraged to adopt practice styles that allow for improved health care provision to these patients–including working with families to determine if increased time for appointments is needed, providing picture/video schedules to help prepare patients to decrease anxiety, identifying other strategies and minimizing triggers specific to the patient, and scheduling patients with consistent providers when possible since familiarity often leads to more success with examinations and better care.
Acknowledgments
This manuscript is dedicated to PD and his family. His family encouraged the publication of his case to increase awareness about the presentation of AVM in children with developmental disabilities and ASD.
References
- Baskaya MK, Jea A, Heros RC, Javahary R, Sultan A (2006) Cerebral Arteriovenous Malformations. Clin Neurosurg 53: 114-115.
- Whigham KB, O’Toole K (2007) Understanding the Neuropsychologic Outcome of Pediatric AVM within a Neurodevelopmental Framework. Cog Behav Neurol 20: 4.
- Millar C, Bissonnette B, Humphreys RP (2005) Cerebral Arteriovenous Malformations in Children. Can J Anaesth 41: 321-331.
- Kondziolka D, Humphreys RP, Hoffman HJ, Hendrick EB, Drake JM (1992) Arteriovenous malformations of the brain in children: a forty year experience.Can J Neurol Sci 19: 40-45.
- Stackhouse T (2010) Motor Differences in the ASD's in Autism: A Comprehensive Occupational Therapy Approach (3rd edn.). In: Kuhaneck and Watling (eds.) Bethesda, MD.
- Yeo JJY, Low SYY, Seow WT, Low DCY (2007) Pediatric de novo cerebral AVM: report of two cases and review of literature. Child’s Nervous System.
- Kilbourn K, Spiegal G, Killory B, Kureshi I (2014) Case Report of a de novo brainstem arteriovenous malformation in an 18-year-old male and review of the literature. NeurosurgRev 37: 685-691.
- Tan W, Baris HN, Burrows PE, Robson CD, Alomari AI, et al. (2007) The Spectrum of Vascular Anomalies in Patients with PTEN mutations: Implicatons for Diagnosis and Management. J Med Genet 44: 594-602.
- Busa T, Milh M, Degardin N, Girard N, Sigaudy S, et al. (2015) Clinical Presentation of PTEN mutations in Childhood in the Absence of Family History of Cowden Syndrome. J Eur Paediatr Neurol Soc 19: 188-192.
- Gupta S, Kanamalla U, Gupta V (2010) Are Incidental Findings on Brain Magnetic Resonance Images in Children Merely Incidental? J Child Neurol Online First.
- Volkmar F, Siegel M, Woodbury-Smith M, King B, McCracken J (2014) American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI): Practice Parameter for the Assessment and Treatment of Children and Adolescents with Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatr 53: 237-257.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences